P668 Differential efficacy of biologic agents for Inflammatory Bowel Disease Unclassified and comparison with Ulcerative Colitis: a propensity score analysis of 4054 patients from the UK IBD BioResource
Kapizioni, C.(1)*;Desoki, R.(1,2);Balendran, K.(1);Lam, D.(1);Shawky, R.(3);Pele, L.(3);Parkes, M.(1);Raine, T.(1);
(1)Cambridge University Hospital, Gastroenterology Department, Cambridge, United Kingdom;(2)Alexandria University, Genetics Department, Alexandria, Egypt;(3)Cambridge University Hospital, IBD BioResource, Cambridge, United Kingdom;
Background
Data on efficacy of biologic drugs in Inflammatory Bowel Diseases Unclassified (IBDU) are lacking as these patients are commonly excluded from randomised controlled trials. In addition, real world data on the use of biologic agents in IBDU are scarce. We explored the effectiveness of first line biologic therapy in patients with IBDU and compared between drugs and with outcomes for patients with Ulcerative Colitis (UC) in the UK IBD BioResource.
Methods
Demographic, disease, treatment and outcome data were retrieved from the UK IBD BioResource, capturing >36,000 subjects across >100 UK centres. Treatment failure was assessed according to persistence on therapy with clinician assessment of treatment success, without the need for treatment change or surgery. Dose-escalation was not considered indicative of treatment failure. Inverse probability of treatment weighting (IPTW) was used to balance groups using a propensity score-weighting approach accounting for baseline patient or disease related clinical characteristics. We compared Kaplan Meier curves for treatment survival using log rank test.
Results
In total, 198 and 3856 evaluable patients with IBDU and UC respectively received first line biologic therapy and were included in the analysis. Median follow up was 2.6 years (IQR 1.1-4.6). In the IBDU group, 104 were female (52.5%), median age at diagnosis was 28 years (IQR 19.7-42) and median disease duration was 10.4 years (IQR 6.7-15.7). The majority of IBDU patients used infliximab (n=143), while the rest used adalimumab (n=42) and vedolizumab (n=13) with no difference in biologic therapy persistence before and after adjustment (before IPTW adjustment, log rank p = 0.062; after IPTW, log rank p = 0.135, Figure 1). No difference was found in biologic therapy persistence between patients with IBDU and UC (before IPTW adjustment, log rank p = 0.581; after IPTW, log rank p = 0.438, Figure 2).
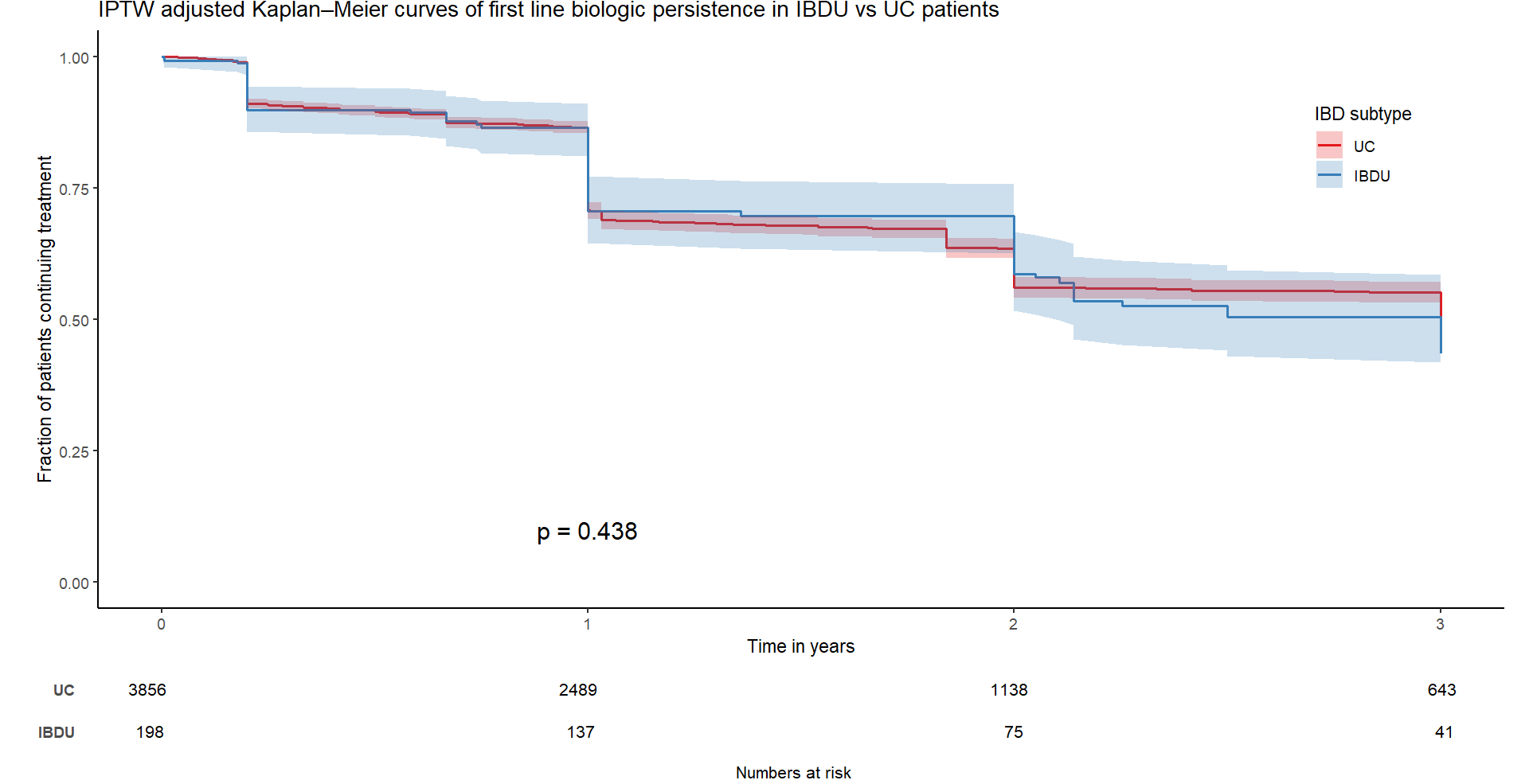
Conclusion
Outcomes for patients with IBDU receiving first line biologic therapy were similar for those observed in patients with UC. This should lend confidence to patients and physicians using biologic therapy for this under-investigated condition. For patients with IBDU, although persistence on vedolizumab was numerically better than for anti-TNFs, this difference did not reach significance and the vedolizumab sample was small. Adalimumab and infliximab appeared to offer similar efficacy for patients with IBDU.


