P670 Usefulness of monitoring the concentration of azathioprine metabolites in the children with inflammatory bowel disease and autoimmune hepatitis
K. Bąk-Drabik1, J. Duda-Wrońska2, D. Dąbrowska-Piechota2, P. Adamczyk3
1Department of Peadiatrics, Faculty of Medical Sciences in Zabrze, Medical University of Silesia- Katowice, Zabrze, Poland, 2Faculty of Medical Sciences in Zabrze, Medical University of Silesia, Katowice Students Association, Department of Peadiatrics, Zabrze, Poland, 3Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Department of Peadiatrics, Katowice, Poland
Background
Azathioprine (AZA) is an immunosuppressive drug, which is metabolised in the liver and kidneys into 6-thioguanine- the form responsible for the therapeutic effect. Despite its anti-inflammatory, antibacterial and immunomodulating properties, azathioprine has also dose-related side effects, such as bone marrow suppression, liver damage and pancreatitis. The purpose of this study was to assess the usefulness of monitoring the concentration of azathioprine metabolites: 6-tioguanine (6-TG) and 6-methylmercaptopurine (6-MMP) in the group of paediatric patients with inflammatory bowel disease (IBD) and autoimmune hepatitis (AIH).
Methods
The clinical data of 46 paediatric patients (24 girls) with IBD and AIH, aged 8–17 years, hospitalised in the Department of Gastroenterology, who had undergone a blood examination for AZA metabolites concentration, were analysed.
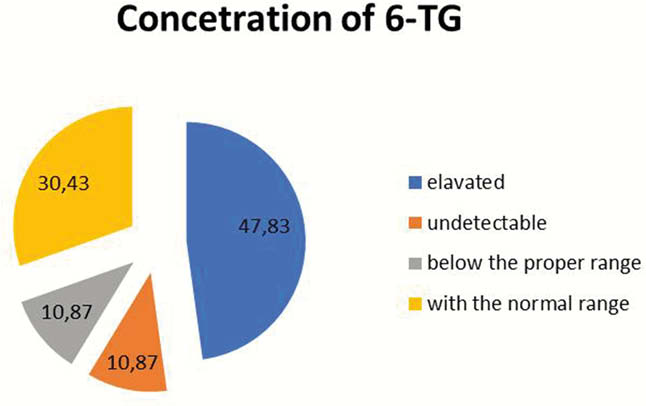
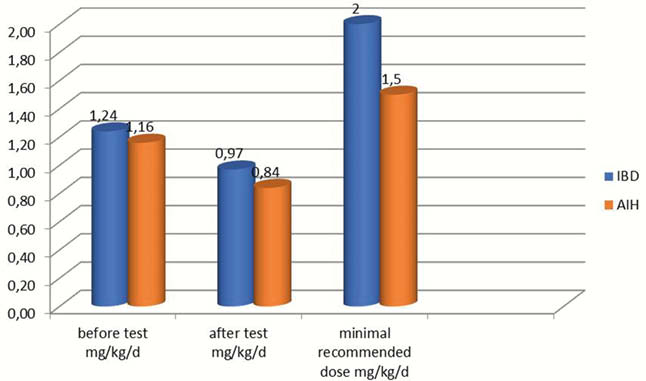
Results
Initial mean dose of azathioprine was 1.23 mg/kg/day in IBD and 1.16 mg/kg/day in AIH. In 30% of patients, the concentrations of 6-TG and 6-MMP were within the normal range. Forty-eight per cent of patients required a dose change due to: elevated 6-TG concentration (32.6%) or underdosage (15.4%). After modification the mean dose was 1.16 mg/kg/day in IBD and 0.85 mg/kg/day in AIH. In 10.7 % of patients, the concentrations of 6-TG and 6 MMP were below the proper range, in the same percentage of patients metabolites were undetectable.
Conclusion
In a significant number of cases monitoring the concentration of AZA metabolites indicated the necessity to reduce the dose of AZA allowing to achieve the therapeutic optimum and prevent serious side effects. Receiving undetectable concentration of metabolites is a sign of non-compliance. The final doses of AZA were found to be lower than the recommended doses. Therapeutic drug monitoring (TDM), which involves measurement of drug or active metabolite levels is a good strategy that can be used to optimise IBD and AIH therapeutics.


