P675 Real world evidence of tofacinitib in ulcerative colitis: short and long-term effectiveness, safety and impact of extraintestinal manifestations and immunomediated diseases
Chaparro Sanchez, M.(1)*;Acosta, D.(1);Rodríguez, C.(2);Mesonero, F.(3);Vicuña, M.(2);Barreiro-de Acosta, M.(4);Fernández-Clotet, A.(5);Hernández Martínez, Á.(6); Arroyo, M.(7);Vera, I.(8); Ruiz-Cerulla, A.(9);Sicilia, B.(10);Cabello Tapia, M.J.(11);Muñoz Villafranca, C.(12);Castro-Poceiro, J.(13);Martínez Cadilla, J.(14);Sierra-Ausín, M.(15);Vázquez Morón, J.M.(16);Montil Miguel, E.(17);Bermejo, F.(18);Royo, V.(19);Calafat, M.(20);González-Muñoza, C.(21);Leo Carnerero, E.(22);Manceñido Marcos, N.(23);Torrealba, L.(24); Alonso-Galán, H.(25);Benítez, J.M.(26);Ber Nieto, Y.(27);Diz-Lois Palomares, M.T.(28);García, M.J.(29);Muñoz, J.F.(30);Armesto González, E.M.(31);Calvet, X.(32); Hernández-Camba, A.(33);Madrigal Domínguez, R.E.(34);Menchén, L.(35);Pérez Calle, J.L.(36);Piqueras, M.(37);Gisbert, J.P.(1);
(1)Hospital Universitario de La Princesa- IIS-Princesa- Universidad Autónoma de Madrid UAM- and CIBEREHD, Gastroenterology Unit, Madrid, Spain;(2)Hospital Universitario de Navarra HUN and Instituto de Investigación Sanitaria de Navarra IdiSNA, Gastroenterology Unit, Pamplona, Spain;(3)Hospital Universitario Ramón y Cajal, Gastroenterology Unit, Madrid, Spain;(4)Hospital Clínico Universitario de Santiago de Compostela, Gastroenterology Unit, Santiago de Compostela, Spain;(5)Hospital Clinic of Barcelona- Institut d'Investigacions Biomèdiques August Pi i Sunyer IDIBAPS and CIBERehd, Gastroenterology Unit, Barcelona, Spain;(6)Hospital Universitario Torrecárdenas, Gastroenterology Unit, Almería, Spain;(7)Hospital Clínico Universitario Lozano Blesa- IIS Aragón and CIBEREHD, Gastroenterology Unit, Zaragoza, Spain;(8)Hospital Universitario Puerta de Hierro Majadahonda, Gastroenterology Unit, Madrid, Spain;(9)Hospital Universitario de Bellvitge, Gastroenterology Unit, L´Hospitalet de Llobregat, Spain;(10)Hospital Universitario de Burgos, Gastroenterology Unit, Burgos, Spain;(11)Hospital Universitario Virgen de las Nieves, Gastroenterology Unit, Granada, Spain;(12)Hospital Universitario de Basurto, Gastroenterology Unit, Bilbao, Spain;(13)Hospital Sant Joan Despí-Moisès Broggi, Gastroenterology Unit, Barcelona, Spain;(14)Hospital Álvaro Cunqueiro. Xerencia Xestión Integrada de Vigo- SERGAS. Grupo de Investigación en Patología Digestiva- IIS Galicia Sur. SERGAS-UVIGO, Gastroenterology Unit, Vigo, Spain;(15)Complejo Asistencia Universitario de León, Gastroenterology Unit, León, Spain;(16)Hospital Universitario Juan Ramón Jiménez, Gastroenterology Unit, Huelva, Spain;(17)Hospital Universitario Miguel Servet, Gastroenterology Unit, Zaragoza, Spain;(18)Hospital Universitario de Fuenlabrada and IdiPAZ, Gastroenterology Unit, Madrid, Spain;(19)Hospital Universitari Son Espases, Gastroenterology Unit, Palma de Mallorca, Spain;(20)Hospital Universitari Germans Trias i Pujol and CIBEREHD, Gastroenterology Unit, Badalona, Spain;(21)Hospital de la Santa Creu i Sant Pau, Gastroenterology Unit, Barcelona, Spain;(22)Hospital Universitario Virgen del Rocío, Gastroenterology Unit, Sevilla, Spain;(23)Hospital Universitario Infanta Sofía, Gastroenterology Unit, Madrid, Spain;(24)Hospital Universitario Dr. Josep Trueta, Gastroenterology Unit, Girona, Spain;(25)Hospital Universitario Donostia and Instituto Biodonostia, Gastroenterology Unit, San Sebastián, Spain;(26)Hospital Universitario Reina Sofía and IMIBIC, Gastroenterology Unit, Córdoba, Spain;(27)Hospital San Jorge, Gastroenterology Unit, Huesca, Spain;(28)Complejo Hospitalario Universitario A Coruña, Gastroenterology Unit, A Coruña, Spain;(29)Hospital Universitario de Valdecilla and Instituto de Investigación Sanitaria Valdecilla IDIVAL, Gastroenterology Unit, Santander, Spain;(30)Hospital Universitario de Salamanca, Gastroenterology Unit, Salamanca, Spain;(31)Hospital San Agustín, Gastroenterology Unit, Avilés, Spain;(32)Hospital Universitari Parc Taulí- Universitat Autònoma de Barcelona and CIBERehd, Gastroenterology Unit, Sabadell, Spain;(33)Complejo Hospitalario Universitario Nuestra Señora de Candelaria, Gastroenterology Unit, Santa Cruz de Tenerife, Spain;(34)Hospital Clínico Universitario de Valladolid, Gastroenterology Unit, Valladolid, Spain;(35)Hospital General Universitario Gregorio Marañón, Gastroenterology Unit, Madrid, Spain;(36)Hospital Universitario Fundación Alcorcón, Gastroenterology Unit, Madrid, Spain;(37)Consorci Sanitari de Terrassa, Gastroenterology Unit, Barcelona, Spain; on behalf of To-ReWard study group
Background
Main aim: To assess the durability of tofacitinib treatment in patients with ulcerative colitis (UC). Secondary aims: To assess the short and long-term effectiveness; the tolerability of tofacitinib in clinical practice; and to evaluate the evolution of extraintestinal manifestations (EIMs) and immunomediated inflammatory diseases (IMIDs).
Methods
Retrospective, multicenter study including UC patients who had received the first tofacitinib dose at least 8 weeks before the inclusion. Patients were followed-up from the first tofacitinib dose to treatment discontinuation or last visit, whichever came first. Only patients with active disease [Partial Mayo Score (PMS)>2] at tofacitinib start were considered in the effectiveness analysis. Clinical effectiveness was based on PMS. In patients who stopped tofacitinib before their last visit, the last observation carried forward method was used to impute missing values at subsequent time points.
Results
408 patients were included (figure 1). 
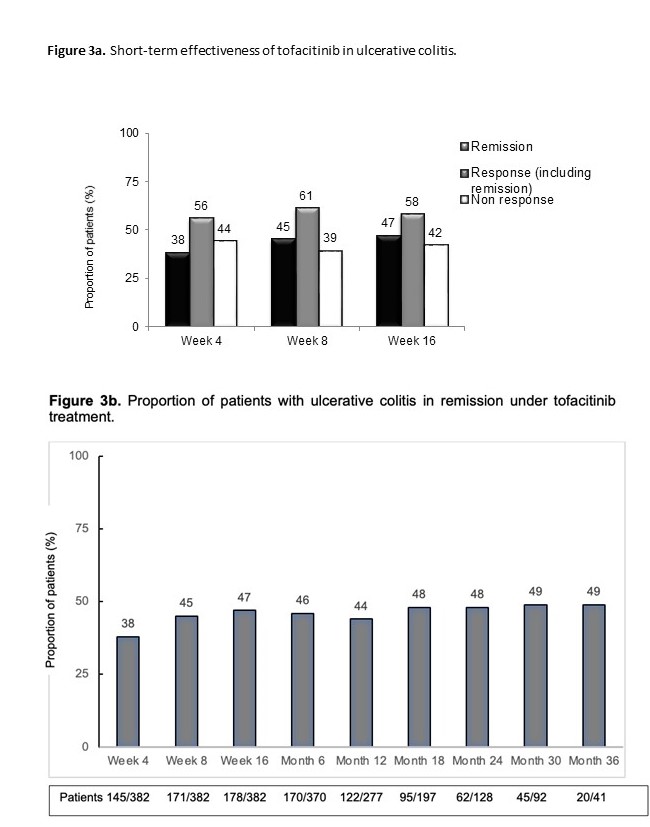
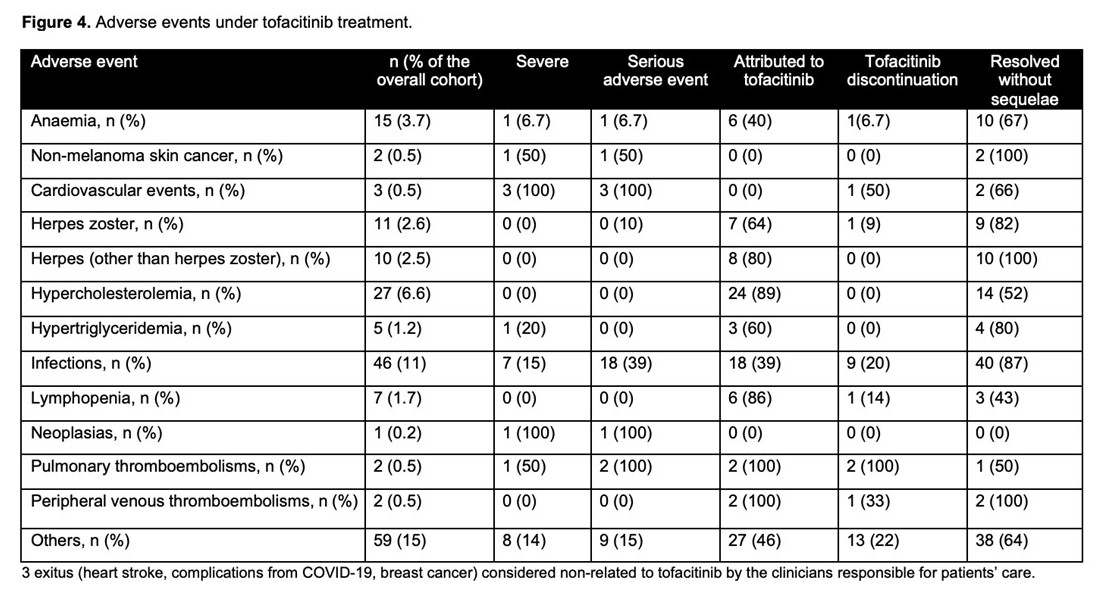
Incidence rate of tofacitinib discontinuation was 41% per patient-year of follow-up. The probability of maintaining tofacitinib is shown in figure 2a. Main reasons for tofacitinib withdrawal were primary non-response (44%) and loss of response (26%). Age at the start of tofacitinib (older) (HR=0.98, 95%CI=0.97-0.99) and the severity of clinical activity were associated with tofacitinib withdrawal (mild vs. remission: HR=1.5, 95%CI=0.5-4; and moderate-severe vs. remission: HR=3.0, 95%CI=1.2-7.4). Short-term effectiveness is shown in figure 3a. To have moderate-severe vs. mild disease activity at baseline (OR=0.2, 95%CI=0.1-0.4) and age at tofacitinib start (older) (OR=1.01, 95%CI=1.002-1.03) were associated with clinical remission at week 8. The probability of maintaining response in shown in figure 2b. Tofacitinib dose was escalated in 55 patients (66%) of those who had lost response, and 82% of them improved (60% regained remission). The proportion of patients on 10 mg b.i.d was over 40% in all timepoints during follow-up. The proportion of patients in clinical remission during follow-up in shown in figure 3b. Adverse events during tofacitinib treatment are summarized in figure 4. There was not any signal of negative impact of tofacitinib on EIM o IMIDs.
Conclusion
Tofacitinib is effective in inducing remission even in highly refractory UC patients. A relevant proportion of patients discontinue the treatment, mostly due to primary failure. Dose escalation is effective to regain response after loss of efficacy. The safety profile is similar to that previously reported


