P681 Effectiveness and safety of oral Tacrolimus salvage therapy for acute severe ulcerative colitis: a retrospective study
Tamir-Degabli, N.(1)*;Cohen, N.A.(2);Hirsch, A.(3);Fishman, S.(2);Ron, Y.(2);Thrum, T.(2);Maharshak, N.(2);
(1)Tel Aviv Sourasky Medical Center- Tel Aviv- Israel, Department of Gastroenterology and Liver Diseases, Tel Aviv, Israel;(2)Tel Aviv Medical Center, Gastroenterology and liver diseases, Tel Aviv, Israel;(3)Tel Aviv Medical Center, Gastroenterology and liver diseases, Tel Avibv, Israel;
Background
Calcineurin inhibitors are a therapeutic option in patients with acute severe ulcerative colitis (ASUC) failing to respond to intravenous steroids. There are limited data regarding the real world effectiveness of tacrolimus in this setting. We describe our experience with tacrolimus in a tertiary IBD center.
Methods
This retrospective study includes patients who were hospitalized at our center with ASUC between 2018-2022 and received tacrolimus salvage therapy. We collected demographic, clinical, and laboratory data. The primary endpoint of this study was clinical response to tacrolimus therapy leading to patient discharge. Secondary endpoints were safety of therapy and long-term effectiveness.
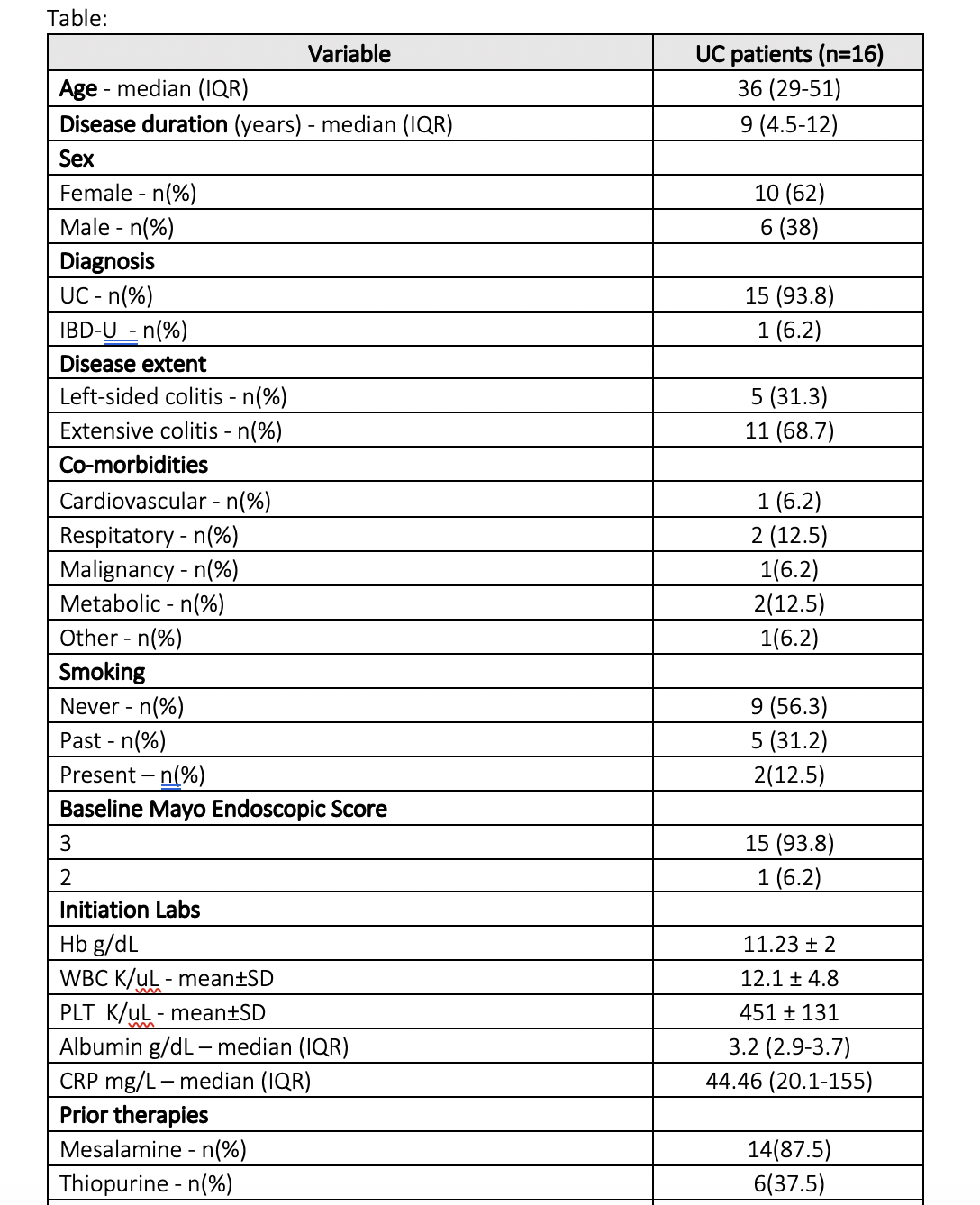
Results
Sixteen patients received tacrolimus therapy; 15 patients with UC and 1 patient with indeterminate colitis. The median age was 36 (interquartile range (IQR) 29-51), median disease duration 9 years (IQR 4.5-12), 10 (69%) were females, 11 (69%) had pan-colitis; 12 (75%) had previous biologic exposure. The median CRP was 44.46 mg/L (IQR 20.1-155), median albumin 3.2 g/dL (IQR 2.9-3.7).
15 patients (94%) responded to tacrolimus and were discharged, 1 patient failed to respond and underwent colectomy. Mean tacrolimus level during induction was 12.48 ng/ml (± 4.5). Following discharge, 1 patient had an exacerbation secondary to poor adherence and underwent colectomy within 2 months. 12 patients were transitioned to biologic therapy (7 vedolizumab, 3 ustekinumab, 2 adalimumab, 1 golimumab) and 1 patient to azathioprine. All 13 patients avoided colectomy with a median follow-up of 5.5 months (IQR 3-12). One patient was lost to follow up. Regarding safety, 10 patients had mild electrolyte disturbance requiring oral supplementation. No other adverse events were observed and no patient discontinued therapy due to adverse events.

Conclusion
Oral tacrolimus salvage therapy shows effectiveness and acceptable safety profile in patients with ASUC. The oral nature of this therapy provides an attractive therapeutic option in this setting.


