P682 Faecal calprotectin and C-reactive protein levels are associated with long-term clinical and endoscopic outcomes: Analysis of the OASIS open-label extension trial of etrasimod for ulcerative colitis
A. Yarur1, M. Chiorean2, J. Zhang3, W. Reinisch4, S. Vermeire5, J. Panés6, L. Peyrin-Biroulet7, B.E. Sands8, C.H. Cabell3, S.U. Naik3, W.J. Sandborn9
1Medical College of Wisconsin, n/a, Milwaukee, USA, 2Virginia Mason Medical Center, n/a, Seattle, USA, 3Arena Pharmaceuticals, n/a, San Diego, USA, 4Medical University of Vienna, n/a, Vienna, Austria, 5University Hospitals Leuven, n/a, Leuven, Belgium, 6Hospital Clinic of Barcelona- DIBAPS- CIBERehd, n/a, Barcelona, Spain, 7Lorraine University, Department of Gastroenterology- INSERM U954, Vandoeuvre-lès-Nancy, France, 8Icahn School of Medicine at Mount Sinai, n/a, New York, USA, 9University of California San Diego, n/a, La Jolla, USA
Background
Reliable biomarkers of ulcerative colitis (UC) disease activity may be useful in clinical trials and practice. Etrasimod is an oral, selective, sphingosine 1-phosphate receptor modulator with efficacy in a 12-week, phase 2, double-blind (DB), randomised, controlled trial in adult patients with moderately-to-severely active UC (OASIS; NCT02447302). Patients who completed the DB study were eligible to enrol in an open-label extension (OLE; NCT02536404) and receive etrasimod 2 mg once daily for up to an additional 34 weeks. The aim of this post-hoc analysis was to assess the correlation of sequential faecal calprotectin (FC) and C-reactive protein (CRP) levels throughout the DB study and OLE with clinical and endoscopic outcomes at end of treatment (EOT) in the OLE.
Methods
In the DB study, patients received etrasimod 1 mg, etrasimod 2 mg or placebo. The OLE evaluable cohort comprised patients who received etrasimod 2 mg throughout the OLE. The modified intention-to-treat (mITT) population comprised patients with non-missing assessments. EOT was the last observation for each patient, occurring at week 46 (OLE week 34) for study completers or at last visit for patients who discontinued or had missing data. Endpoints were modified Mayo Clinic score (mMCS; range 0–9; including endoscopy, rectal bleeding [RB], and stool frequency [SF]); clinical remission (endoscopic subscore ≤1 [with absence of friability], RB ≤1, and SF score ≤1 with ≥1 point decrease from DB baseline); clinical response (clinical remission or decrease in mMCS of ≥2 points and ≥30% decrease from DB baseline, with either a RB decrease of ≥1 or RB score of ≤1); and endoscopic improvement (subscore ≤1). FC and CRP were measured longitudinally to EOT. Comparisons between subgroups were assessed with a Wilcoxon rank-sum test (2-sided
Results
The evaluable cohort included 105 patients, 31 of whom received etrasimod 2 mg throughout both DB and OLE periods. At EOT 70%, 35% and 45% of patients in the mITT evaluable cohort had clinical response, clinical remission and endoscopic improvement, respectively. Differences in FC and CRP levels between patients with and without clinical remission at EOT are shown in Figures 1 and 2, respectively for patients who received etrasimod 2 mg throughout both the DB and OLE periods. Correlation analyses of FC and CRP with clinical (mMCS) and endoscopic disease activity and with each other are shown in Table 1.
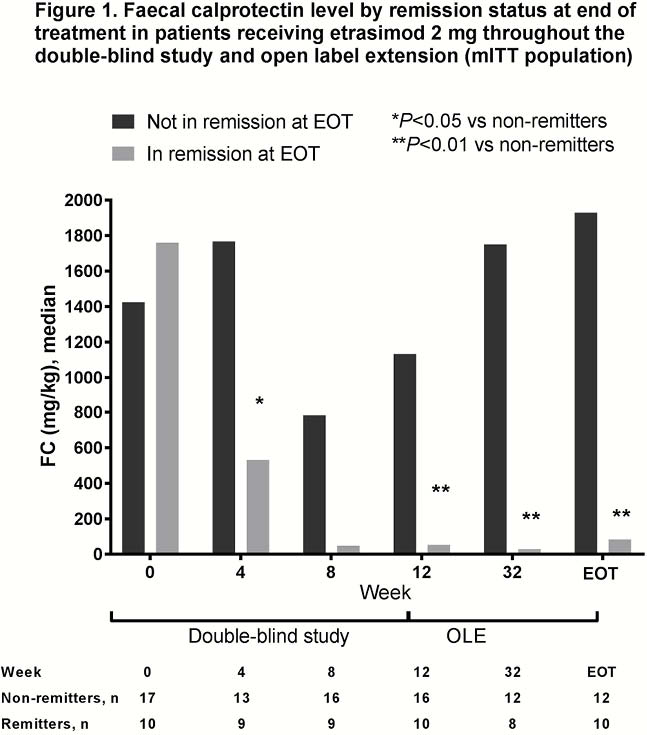
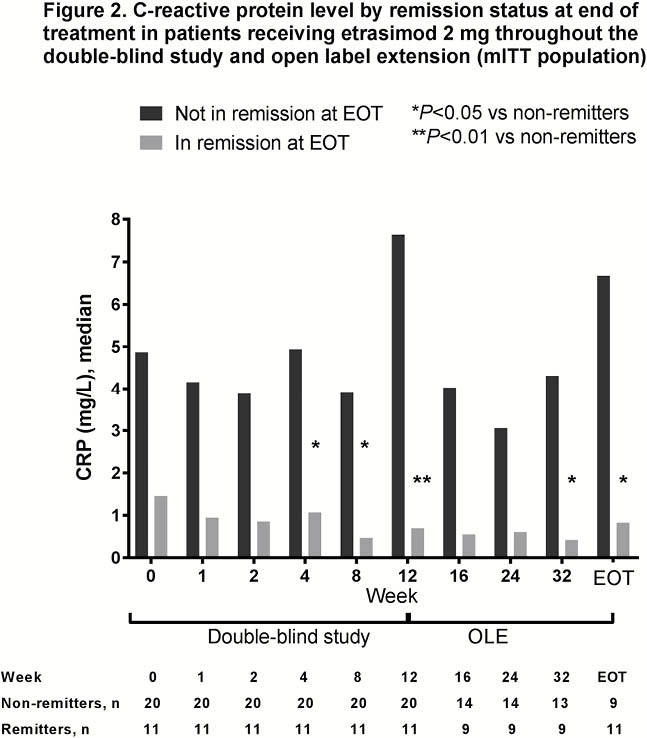
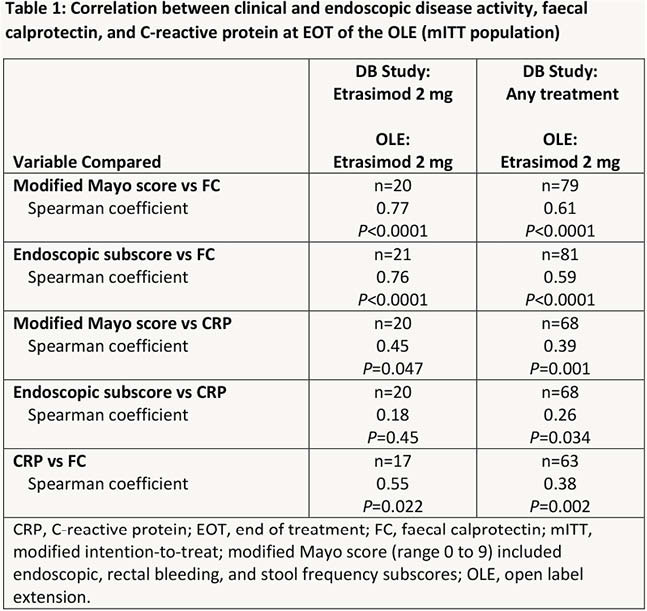
Conclusion
FC and CRP appear to correlate with clinical and endoscopic outcomes over long-term treatment with etrasimod. Additional validation is needed to determine their utility in treat-to-target management strategies.


