P683 Discontinuation of infliximab treatment: a comparison between IBD patients who retransitioned to originator and those who remained on biosimilar
Meijboom, R.(1);Gardarsdottir, H.(2);Becker, M.(1);Movig, K.(3);Egberts, T.(2);Giezen, T.(1);
(1)Pharmacy Foundation of Haarlem Hospitals- Haarlem- the Netherlands, Pharmacy Foundation of Haarlem Hospitals- Haarlem- the Netherlands, Haarlem, The Netherlands;(2)Utrecht Institute for Pharmaceutical Sciences, Division of Pharmacoepidemiology & Clinical Pharmacology, Utrecht, The Netherlands;(3)Medisch Spectrum Twente, Department of Clinical Pharmacy, Enschede, The Netherlands;
Background
Since the introduction of infliximab biosimilars, many patients with inflammatory bowel disease (IBD) transitioned from originator (Remicade) to an infliximab biosimilar, mainly for cost containment. Studies showed that, despite proven biosimilarity, 2 to 16.5% of patients who transition to biosimilar, retransition to infliximab originator (stopped biosimilar and re-initiated originator), mainly due to increased disease activity or (subjective) adverse events. It is unclear whether this unsatisfactory treatment response is related to the product or to the patient and his/her disease.
This study aimed to compare the risk of infliximab treatment discontinuation between patients who retransitioned from infliximab biosimilar to originator and patients who remained on infliximab biosimilar.
Methods
IBD patients treated at the Medisch Spectrum Twente or Spaarne Gasthuis who transitioned from Remicade to an infliximab biosimilar between January 2015 and September 2019 were eligible for inclusion. Patients who retransitioned to Remicade were included in the retransitioning cohort and matched with up to 3 patients who remained on biosimilar (biosimilar cohort). Patients were matched on: hospital, calendar date of biosimilar transitioning and duration of biosimilar treatment.
Patients were followed until discontinuation of infliximab (>16 weeks no administration, or switch to another biological). Risk of infliximab discontinuation (overall discontinuation and stratified into switching to another biological or discontinuation without switching) was compared using a (adjusted) conditional Cox proportional hazards model.
Results
The retransitioning cohort comprised 42 patients and the biosimilar cohort comprised 120 patients. Baseline characteristics of the cohorts were similar, but dosing interval was shorter in the retransitioning cohort (44 versus 56 days). Infliximab treatment discontinuation was 24.6% in the retransitioning cohort compared to 6.7% in the biosimilar cohort at 12 months (p<0.01) (Figure 1). Patients in the retransitioning cohort had a more than twofold risk (HR 2.5, 95%CI 1.3-5.1) for discontinuing infliximab treatment compared to patients in the biosimilar cohort. When analyzing the type of discontinuation, patients in the retransitioned cohort had a fivefold risk for switching to another biological (HR 4.8, 95%CI 0.6-37.0) and over a twofold risk for discontinuing without switching (HR 2.4, 95%CI 1.1-5.5) (Table 1).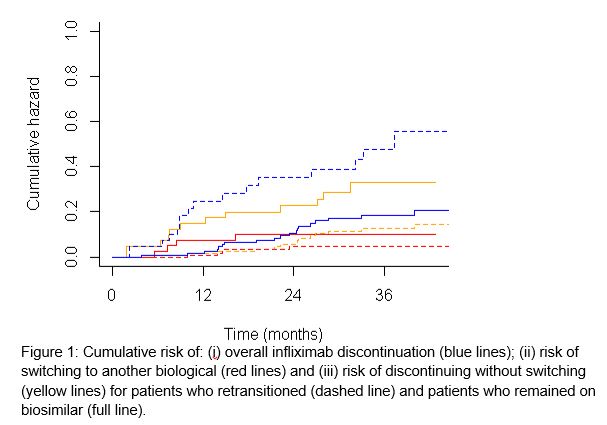

Conclusion
Retransitioned patients have an increased risk for infliximab discontinuation, including both switching to another biological or discontinuing without switching. Our results indicate that retransitioning is mainly patient or disease related and less likely to be product related.


