P683 Infliximab, adalimumab and vedolizumab levels are not altered by pregnancy progression in IBD patients and neonatal vedolizumab levels are lower than in mothers: Results from the PICCOLO study
E. Flanagan1, P.R. GIbson2, O. Rosella3, A. Ross4, S.J. Bell4, The PICCOLO Study
1Department of Gastroenterology, St Vincent’s Hospital Melbourne, Fitzroy, Australia, 2Department of Gastroenterology, Alfred Health, Monash, Australia, 3Department of Gastroenterology, Monash University, Melbourne, Australia, 4Department of Gastroenterology, St Vincent’s Hospital Melbourne, Melbourne, Australia
Background
Optimal timing for the last intrapartum dose of biologic agents is controversial. We established that cord blood levels of anti-TNF agents in exposed infants are greater than maternal levels,1 and another study with limited observations suggested maternal infliximab (IFX) levels increased in pregnancy but adalimumab (ADA) levels did not.2 The effect of pregnancy on vedolizumab (VDZ) levels, and placental transfer and time to clearance of VDZ in exposed infants remain undefined.
Methods
We performed a prospective observational study of maternal anti-TNF and VDZ levels pre-conception, in each trimester of pregnancy, at delivery and post-partum where possible. Women with IBD on IFX, ADA or VDZ who were pregnant or planning pregnancy were recruited. Serum trough IFX and ADA levels were measured by ELISA (Q-INFLIXI and Q-ADA (Matriks Biotek, Turkey) or Promonitor (Grifols, Spain)) and VDZ levels by ELISA (Theradiag, France). Infant VDZ levels were measured from the umbilical cord at delivery and at around 6–8 weeks and 3 months to assess time to clearance.
Results
Forty-nine patients (24 IFX, 14 ADA, 11 VDZ) with at least two observations on stable dosing were included. The median number of levels per patient was 3 (range 2–5).
Box-plots of trough drug levels before pregnancy (Pre), during each trimester (T1, T2, T3) and post-partum (PP)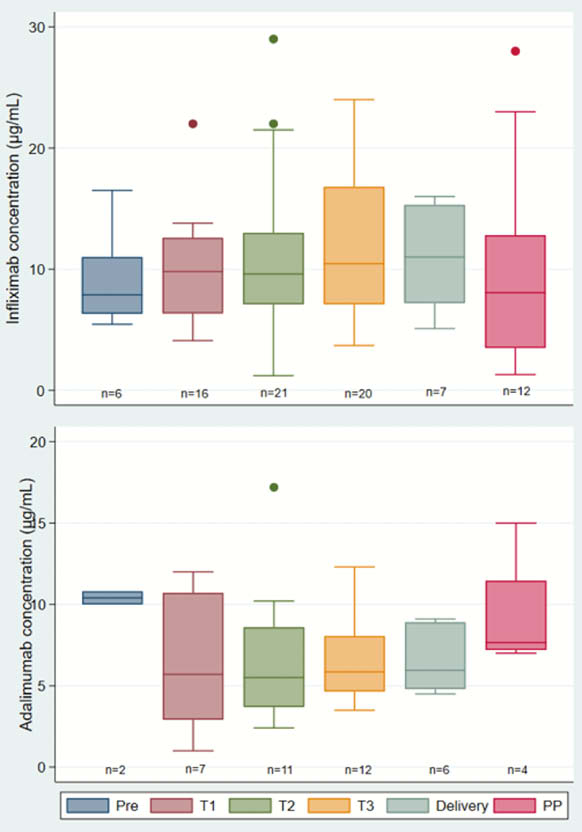
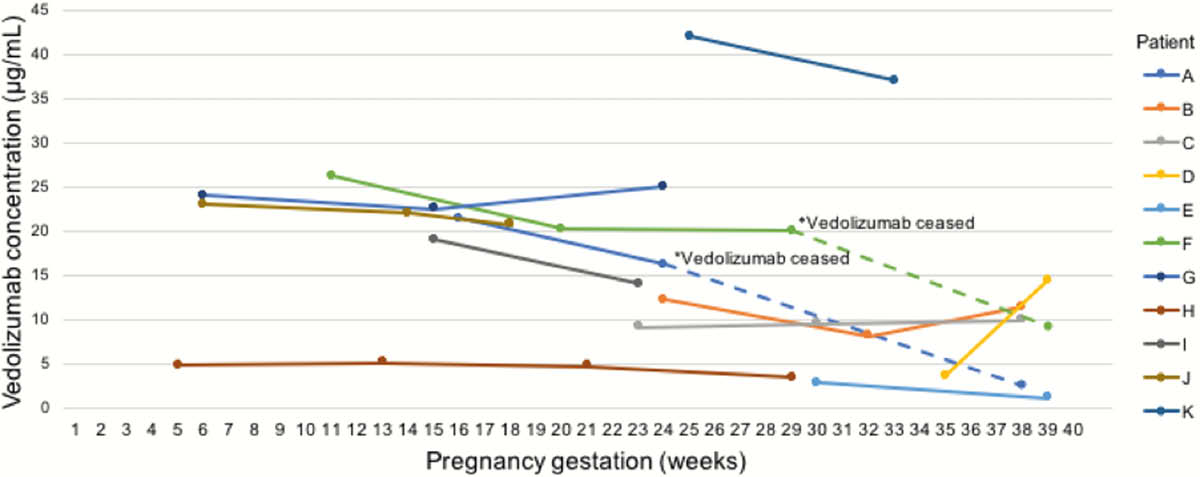
Conclusion
The stability of maternal levels of IFX, ADA and VDZ during pregnancy indicate that intrapartum dosing adjustment and routine therapeutic drug monitoring for patients in remission are not needed. Unlike anti-TNF, infant VDZ levels were lower in cord blood than in mothers and were cleared by 15 weeks.
Julsgaard M
Seow CH


