P684 The effect of tofacitinib on serum lipids and cardiovascular safety in patients with ulcerative colitis: updated results from the tofacitinib ulcerative colitis clinical programme
B.E. Sands1, P.R. Taub2, B.G. Feagan3, A. Armuzzi4, A.O. Damião5, N. Lawendy6, G. Solano7, K. Kwok8, J. Woolcott6, L. Salese6, C. Su6
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York- NY, USA, 2Division of Cardiovascular Medicine- UC San Diego School of Medicine, La Jolla, California, USA, 3Robarts Clinical Trials- Western University, London, Ontario, Canada, 4IBD Unit- Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy, 5Department of Gastroenterology, University of São Paulo USP, São Paulo, Brazil, 6Pfizer Inc., Collegeville, Pennsylvania, USA, 7Pfizer Inc., San José, Costa Rica, Costa Rica, 8Pfizer Inc., New York, New York, USA
Background
Tofacitinib is an oral, small-molecule JAK inhibitor for the treatment of ulcerative colitis (UC). In the tofacitinib UC clinical programme, the majority of patients did not have cardiovascular (CV) risk factors at baseline (BL), and increases in lipid levels occurred primarily during induction and remained elevated to Week 61 during maintenance; lipid ratios were relatively unchanged.1 Here, we present updated results of lipid levels in the ongoing, open-label, long-term extension (OLE) study (NCT01470612) and major adverse CV events (MACE) in the Phase 3/OLE tofacitinib UC clinical programme.
Methods
Lipid levels were assessed at multiple time points, and changes from OLE study BL to Month 48 in the OLE study were evaluated. Lipid-lowering agent (LLA) use (proportion of patients) and adjudicated MACE (proportion of patients and incidence rate [IR; unique patients with events per 100 patient-years] with 95% confidence interval [CI]) were reported in patients with UC in two Phase 3, 8-week, placebo-controlled induction studies (OCTAVE Induction 1 and 2; NCT01465763, NCT01458951), a Phase 3, 52-week, placebo-controlled maintenance study (OCTAVE Sustain; NCT01458574) and the OLE study (data as of May 2019; database not locked).
Results
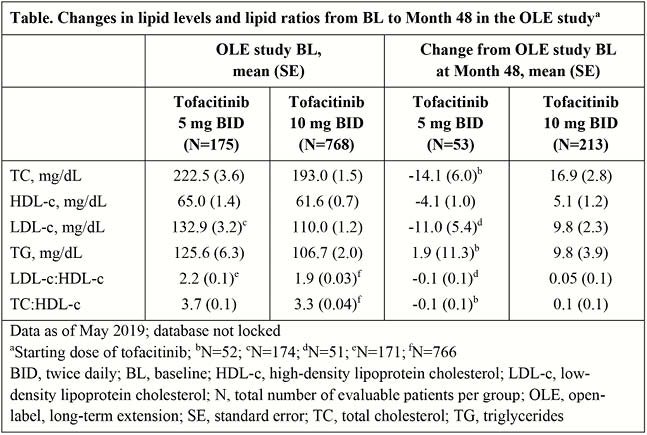
No major changes in total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), triglycerides, LDL-c:HDL-c and TC:HDL-c from OLE study BL were observed at Month 48 of the OLE study (Table). In the Phase 3/OLE programme (
Conclusion
At Month 48 of the OLE study, lipid levels and ratios remained generally unchanged from OLE study BL following tofacitinib treatment. MACE were infrequent, with the IR remaining stable since the previous report.1 Limitations include low patient numbers and short tofacitinib exposure duration. Longer-term observation studies will further assess risk.
Sands BE


