P685 Strong Response to SARS-CoV-2 Vaccine Additional Doses among Patients with Inflammatory Bowel Diseases
Kappelman, M.(1);Weaver, K.(2);Zhang, X.(1);Chun, K.(3);Long, M.(2);
(1)University of North Carolina, Division of Pediatric Gastroenterology- Department of Pediatrics, Chapel Hill, United States;(2)University of North Carolina, Medicine, Chapel Hill, United States;(3)Labcorp, Esoterix, Calabasas, United States; PREVENT-COVID Study Group
Background
Current recommendations in many countries support additional COVID-19 vaccine doses in patients with inflammatory bowel disease (IBD) who are treated with immunosuppressants, yet real-world data on the effectiveness and safety of additional vaccine doses is lacking. We sought to quantify the humoral immune response to an additional vaccine dose in patients with IBD.
Methods
We performed a direct-to-patient, internet-based cohort study of patients with IBD in the United States who have received any SARS-CoV-2 vaccine granted EUA. Participants completed baseline and follow-up surveys and had blood work obtained approximately 8 weeks following completion of the initial vaccination series and 6 weeks following administration of an additional vaccine dose. We performed quantitative measurement of anti-receptor binding domain (RBD) IgG antibodies specific to SARS-CoV-2 using the LabCorp Cov2Quant IgG™ assay.
Results
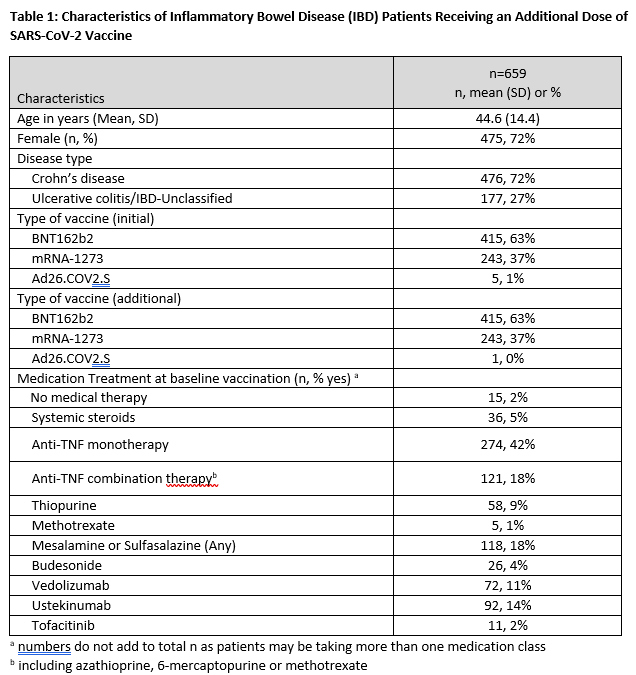
A total of 659 participants were included [415 participants (63%) initially received BNT162b2, 243 participants (37%) initially received mRNA-1273, and 5 participants (1%) initially received Ad26.COV2.S]. Demographic, clinical, and treatment characteristics of the study population are provided in Table 1. Over 98% of those receiving an initial mRNA vaccine received the same type additional dose. Whereas 93% had detectable antibody after the initial vaccination series, 99.5% had detectable antibodies following an additional dose. Mean (SD) increase in antibody level was 61 ug/mL (103) in those receiving BNT162b2 and 78 ug/mL (143) for those receiving mRNA-1273 (Figure 1). Importantly, of 47 of patients without initial antibody response, 45 (96%) had detectable antibodies following an additional dose. Additional vaccination was generally well tolerated in this population, with 44%, 24%, 25%, and 6% reporting no, mild, moderate, and severe side effects respectively.
Conclusion
These findings demonstrate robust immunogenicity to additional doses of SARS-CoV-2 vaccine, even amongst patients with undetectable antibody following the initial series. Adverse event rates were low. These data can be used to inform vaccine decisions in patients with a broad array of immune-medicated conditions frequently managed by immunosuppression.