P699 Ustekinumab therapy after biologic failure in patients with Crohn’s Disease – A real-world single centre experience
Majumder, S.(1,2,3)*;Bazarova, A.(4);Lorenzo Parigi, T.(1,3); Love , M.(5);Davis , J.(3);Ghosh, S.(6); Iacucci , M.(1,3,7);N. Shivaji, U.(1,3,7);
(1)University of Birmingham, Institute of Immunology and Immunotherapy, Birmingham, United Kingdom;(2)India Institute- University of Birmingham, Fellowship, Birmingham, United Kingdom;(3)University Hospitals Birmingham NHS Foundation Trust, Gastroenterology, Birmingham, United Kingdom;(4)Institute for Biological Physics University of Cologne, Biological Physics, Cologne, Germany;(5)University Hospitals Birmingham NHS Foundation Trust, Gastroenterology, Gastroenterology, United Kingdom;(6)College of Medicine and Health- University College Cork, APC Microbiome, Cork, Ireland;(7)National Institute for Health Research NIHR Birmingham Biomedical Research Centre- University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Institute of Immunology and Immunotherapy, Birmingham, United Kingdom;
Background
Patients with Crohn’s disease (CD) often require multiple biological therapies due to loss of response. Anti-TNF drugs are generally used as first-line biologics followed by a switch of class. Ustekinumab (UST), an IL-12/23p40 antagonist is used often after anti-TNF failure. The aim of the study was to report on the outcomes of UST therapy with a median follow-up period of nearly 24 months.
Methods
All CD patients who commenced UST therapy were identified from EMR at a tertiary referral centre between January 2017 and December 2021. All relevant demographic and clinical data were collected. Data on clinical response (defined as a downgrade in disease activity based on clinician assessment & biochemical parameters), steroid-free duration, and long-term response to UST at 52 and 104 weeks were recorded. The response was assessed clinically and supported by biomarkers, cross-sectional imaging, colonoscopic data, and sustained maintenance of UST. The statistical analysis was carried out using the IBM® SPSS® Statistics software package Version: 28.0.0.0
Results
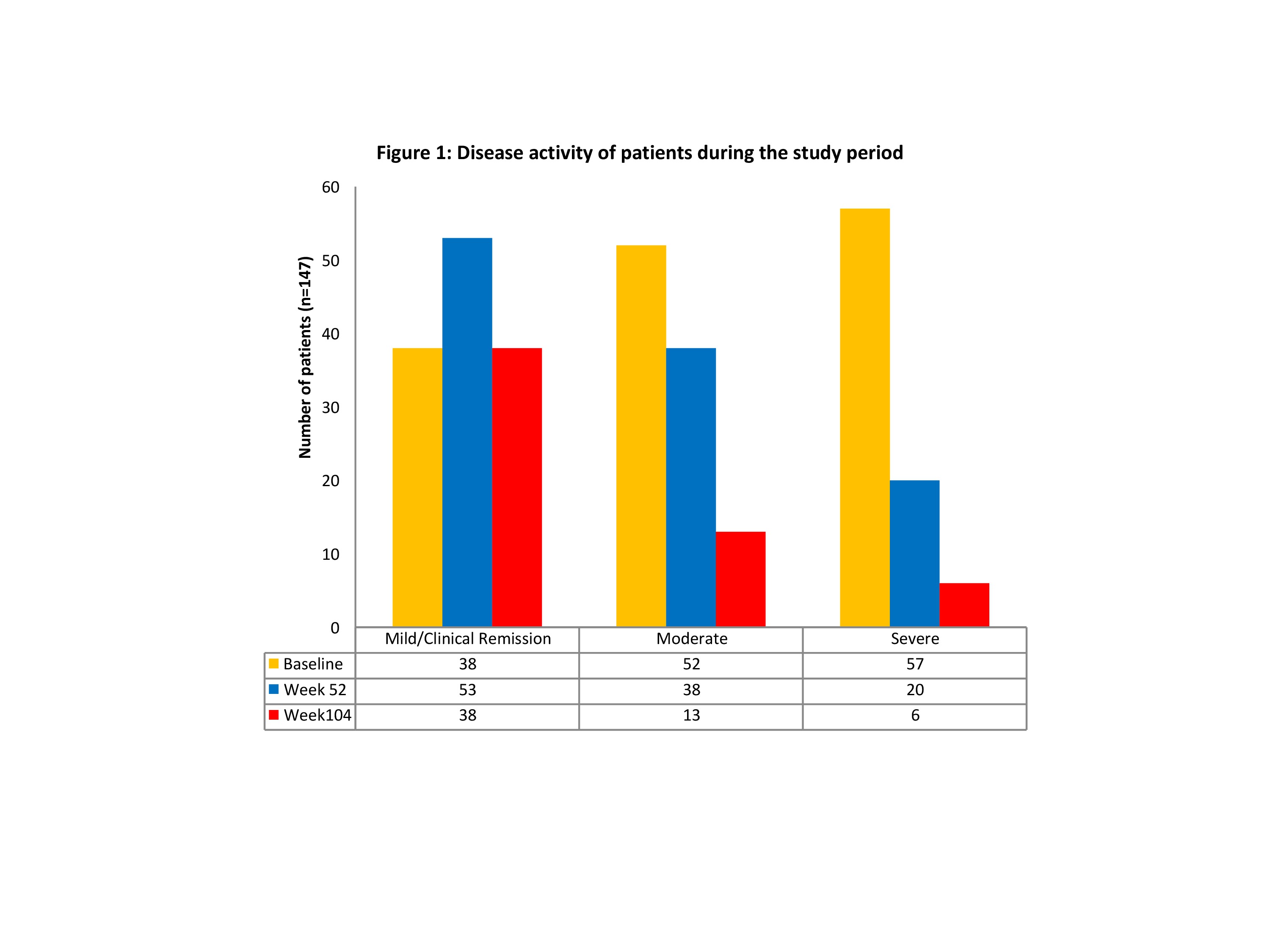


A total of 147 CD patients (M=65, 44%; median age 38years) were included in the analysis with a median follow-up period of 24 months (range 5-67 months). 82 (56%) patients had stricturing (B2), penetrating (B3) and perianal phenotype at baseline, and 50 (34%) had undergone the previous resection/s. 109 (75%) had documented moderate to severe disease activity and 139 (95%) patients were exposed to at least one biologic prior to treatment with UST. A total of 34 (23%) patients were on concomitant thiopurines at the start of treatment. Among 147 patients in our cohort, 143 (97%) showed clinical response to UST and remained on treatment at the end of the follow-up period. The distribution of patients as per disease activity at baseline, 52 and 104 weeks is illustrated in Figure 1. An improvement in haemoglobin levels was observed post-therapy with UST, which was statistically significant at 104 weeks (p<0.001), with a corresponding significant reduction in faecal calprotectin levels (median reduction from 976 mcg/g 333 mcg at 104w; p<0.001). Only 13(8.8%) patients had side effects that were directly attributed to UST therapy.
Conclusion
UST is an effective therapeutic option in patients with CD who have failed previous biologic therapy. In our cohort with a large proportion of patients with complicated and refractory disease, clinical response was observed regardless of their previous exposure status to biologics, and disease phenotype. UST appears to be well tolerated with a reasonable safety profile. The consistent response rates even after prolonged treatment periods mean that clinicians could safely continue UST for longer durations, where options are limited.


