P704 The biennial direct pharmaceutical costs per treatment with biologics for the inflammatory bowel disease in Greece: A comparative calculation study
C. Liatsos1, A. Papaefthymiou1, N. Kyriakos1, M. Giakoumis1, M. Tzouvala2, M. Doulberis3, C. Mavrogiannis4, J. Kountouras5
1Department of Gastroenterology, 401 General Military Hospital of Athens, Athens- Attica, Greece, 2Department of Gastroenterology, Agios Panteleimon General Hospital, Nikaia- Attica, Greece, 3Department of Gastroenterology and Hepatology, University of Zurich, Zurich, Switzerland, 4Faculty of Nursing- School of Public Health, National and Kapodistrian University of Athens, Athens- Attica, Greece, 5Department of Internal Medicine- Second Medical Clinic, Ippokration Hospital- Aristotle University of Thessaloniki, Thessaloniki- Macedonia, Greece
Background
Inflammatory bowel disease (IBD), as a chronic disease with relatively high prevalence worldwide, has undoubtedly resulted in a notable economic burden on health care systems globally. The IBD treatment with biologics (IBD-BT) seems quite complex with various strategies to induce and maintain remission and balance against long-term complications. IBD-BT costs have never been estimated in detail so far in Greece, especially during such a severe 10-years financial crisis experience.
Methods
Direct pharmaceutical costs for one and two years, both for induction and maintenance, adult treatment diagnosed with Crohn’s disease (CD-BT) or Ulcerative colitis (UC-BT) were estimated. For intravenous agents, the hospital drug prices and one day admission costs were calculated, whereas for subcutaneous biologics the retail ones. It was taken for granted that all patients were fully responders and after the approved induction scheme continued with the standard maintenance strategy. Prototype and biosimilar drug prices were also assessed where available. More specifically, when considering biosimilars, the most affordable one was included to our analysis. Each drug price was estimated based on the data collected from the 2019 Greek official electronic national publication on drug therapy of the Greek national organisation for medicines.
Results
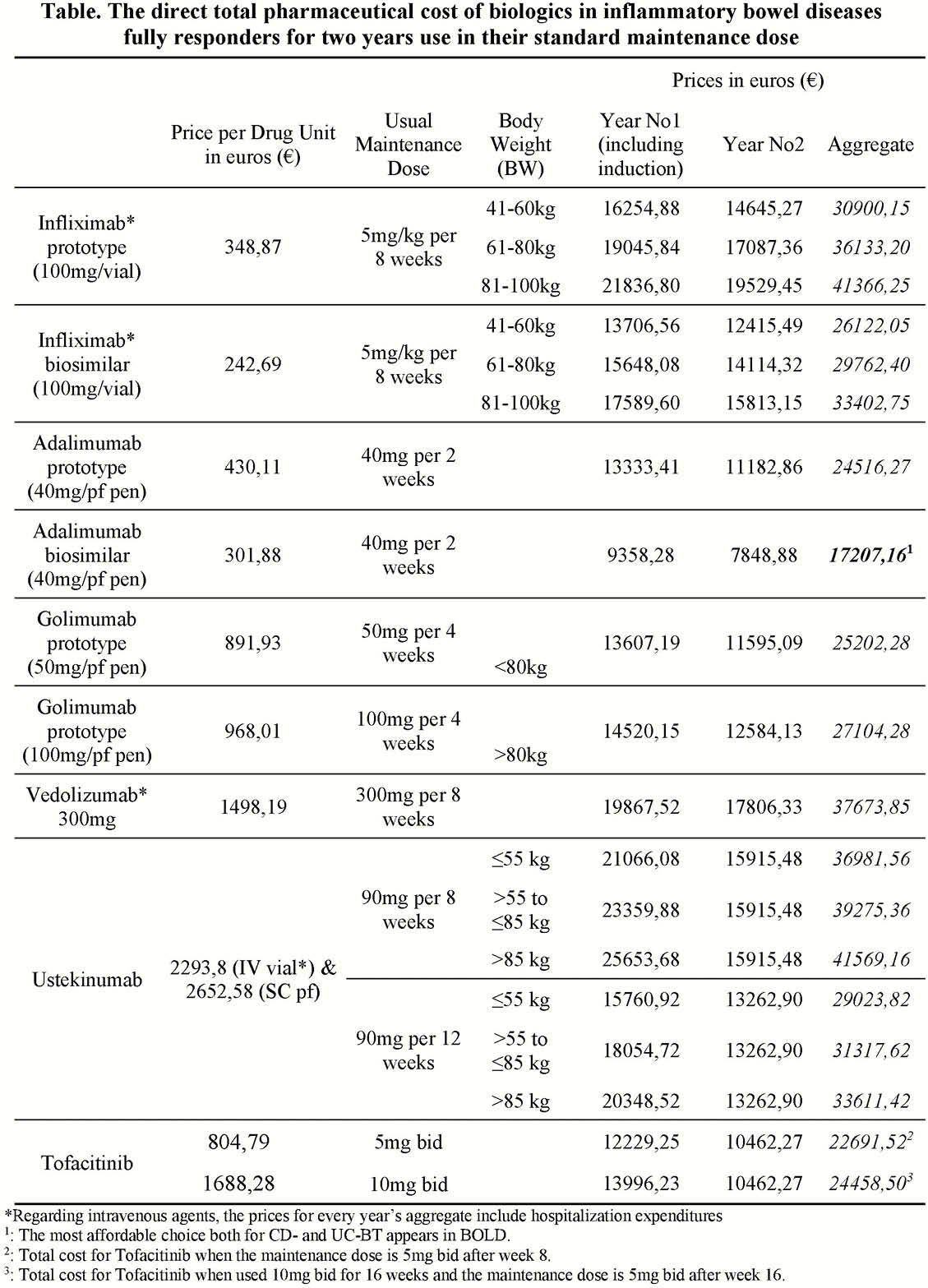
Table shows the costs in euros of each IBD-BT scheme. The biosimilar adalimumab was proved as the most affordable choice both for CD- and UC-BT. The second most affordable choice for CD revealed to be the prototype Adalimumab and respectively for UC the recently introduced tofacitinib, in the maintenance dose of 5 mg bid after week 8 (with a slight burden when the more intensive scheme with Tofacitinib 10 mg bid for 16 weeks is necessary). The most expensive strategies include Ustekinumab 90 mg (per 8 weeks for body weight—BW > 55 to ≤85 kg and >85 kg) and the prototype Infliximab 5 mg/kg (per 8 weeks for BW>81 kg), whereas Vedolizumab remains expensive regardless BW. It is worthwhile to mention that the hospitalisation expenditures (563€) raise the costs of intravenous agents when compared with the subcutaneous ones.
Conclusion
The biennial direct pharmaceutical costs for the approved IBD-BT schemes both for induction and maintenance phases in fully responders were estimated thoroughly for the first time in Greece. These results should motivate Governments and European Union policymakers in order to promote cost-benefit and cost-utility studies to offer the best patients’ benefit by evaluating and deciding the most suitable regimen with respect to biologic prices, adverse effects, hospitalisation expenditures, IBD complications and recurrences.


