P712 Association of histologic-endoscopic mucosal healing after ustekinumab induction or maintenance therapy with 2-year outcomes in the UNIFI Phase 3 study in ulcerative colitis
K. Li1, F. Yang1, C. Marano1, H. Zhang2, W.J. Sandborn3, B.E. Sands4, B.G. Feagan5, D.T. Rubin6, L. Peyrin-Biroulet7, J.R. Friendman1, G. De Hertogh8
1UNIFI Investigators, 1Janssen Research & Development- LLC, Immunology, Spring House, USA, 2Janssen Research & Development- LLC, Clinical Biostats, Spring House, USA, 3University of California San Diego, Gastroenterology, La Jolla, USA, 4Icahn School of Medicine at Mount Sinai, Gastroenterology, New York, USA, 5Robarts Research Institute, Robarts Clinical Trials, London, Canada, 6University of Chicago Medicine, Gastroenterology, Chicago, USA, 7Nancy University Hospital- Université de Lorraine, Gastroneterology, Nancy, France, 8University Hospitals KU Leuven, Pathology, Leuven, Belgium
Background
Ustekinumab (UST) is an effective therapy for moderate-to-severe ulcerative colitis (UC). Histological and endoscopic improvement of mucosa after induction are associated with clinical remission and steroid-free clinical remission at maintenance Week 44. The association of histological-endoscopic mucosal healing after UST induction or maintenance therapy with 2-year outcomes in moderate-to-severe UC is not known.
Methods
In the UNIFI study of UST in moderate-to-severe UC, histological-endoscopic mucosal healing was defined as achieving both endoscopic improvement (Mayo endoscopy subscore ≤1) and histological improvement (i.e., neutrophil infiltration in <5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue; based on the Geboes score). Associations of mucosal improvement after induction (irrespective of treatment) or UST maintenance with long-term efficacy of UST, disease severity, inflammation level, and dose adjustment were evaluated up to Week 92 in the long-term extension (LTE) phase of UNIFI. Analysis was conducted in patients who were randomised to receive UST maintenance therapy and continued with UST in LTE. Tests with
Results
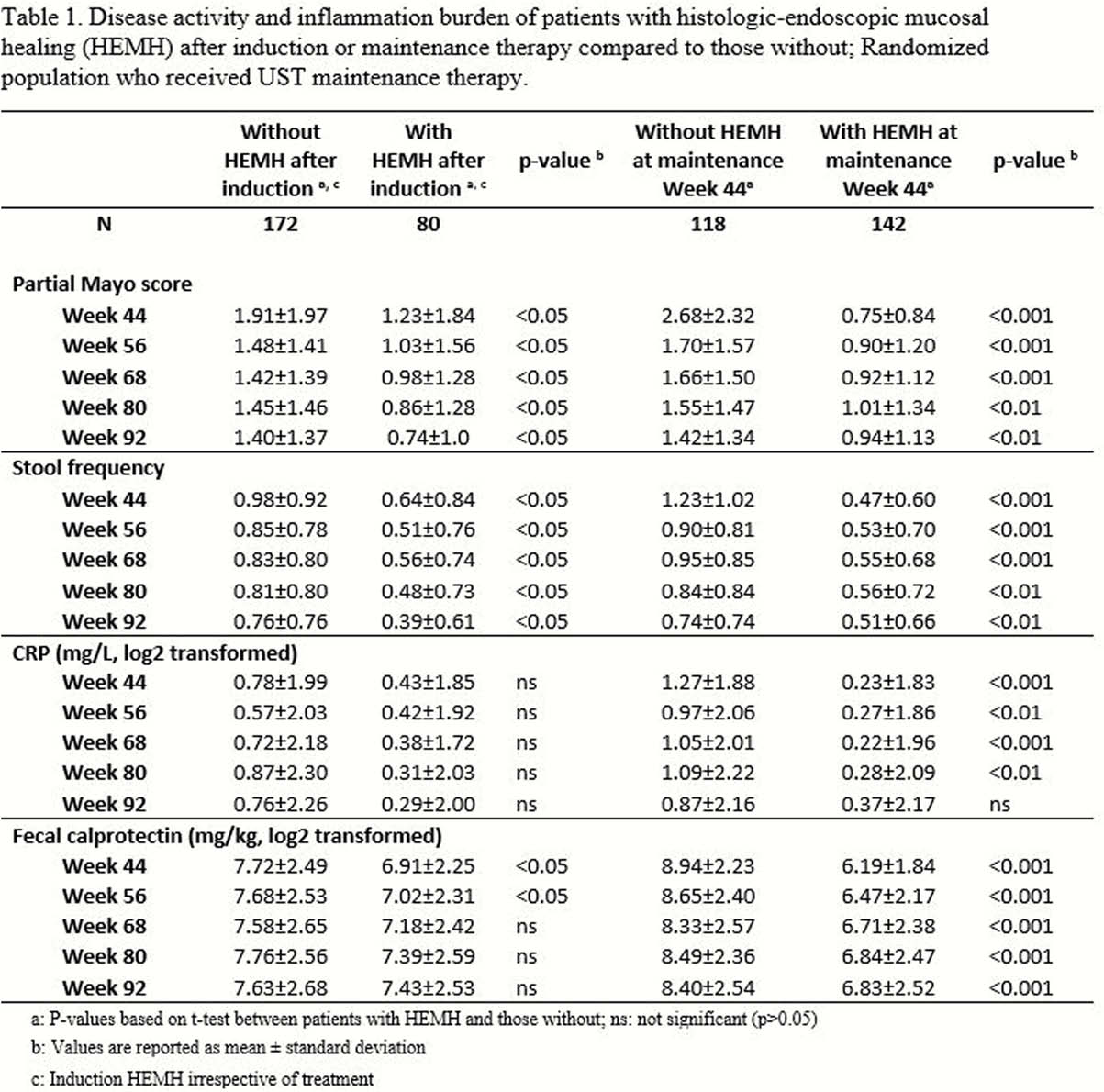
Patients with histological-endoscopic mucosal healing after induction had significantly lower disease activity (partial Mayo score and stool frequency) and a trend for lower inflammation measured by CRP and faecal calprotectin at maintenance Week 44 than those without induction mucosal healing (Table 1). These improvements in disease activity were retained through LTE Week 92, with patients with induction histological-endoscopic mucosal healing showing a continuous reduction of disease activity. Significantly lower disease activity, CRP, and faecal calprotectin were also observed through LTE among patients with histological-endoscopic mucosal healing after UST maintenance compared with those without (Table 1). Patients with histological-endoscopic mucosal healing after induction remained on treatment longer than those without (
Conclusion
Early macroscopic and microscopic improvement of the mucosa is an indicator of positive long-term clinical outcomes and reductions in inflammatory burden.


