P713 Effects of vedolizumab on health-related quality of life, work productivity and patient concerns in patients with ulcerative colitis and Crohn’s disease in the UK and Ireland: OCTAVO cohort 2
G. Parkes1, A. Akbar2, I. Beales3, M. Buckley4, T. Creed5, S. Din6, A. Fraser5, N. Plevris7, S. Meadowcroft8, G. Owen9, N. Heggs8, OCTAVO
1Department of Gastroenterology, The Royal London Hospital- Barts Health NHS Trust, London, UK, 2Gastroenterology Department, St Mark’s Hospital and Academic Institute-, London, UK, 3Department of Gastroenterology, Norfolk and Norwich University Hospitals NHS Foundation Trust, Norwich, UK, 4Department of Gastroenterology, Mercy University Hospital, Cork, Ireland, 5Gastroenterology Department, Bristol Royal Infirmary- University Hospitals Bristol NHS Foundation Trust, Bristol, UK, 6Department of Gastroenterology, University Hospitals of Derby and Burton NHS Foundation Trust- Royal Derby Hospital, Derby, UK, 7Gastroenterology, The Edinburgh IBD Unit- Western General Hospital, Edinburgh, UK, 8Medical Department, Takeda UK Ltd, London, UK, 9Market Access, Takeda UK Ltd, London, UK
Background
Patients with inflammatory bowel disease (IBD) experience substantial impairment in health-related quality of life (HRQoL), therefore HRQoL endpoints are considered important measures of treatment outcome. OCTAVO evaluated the effects of vedolizumab on HRQoL, work productivity and patient concerns in IBD patients in the UK and Ireland.
Methods
OCTAVO is an ongoing multicentre, observational study to evaluate the impact of vedolizumab on two cohorts. Cohort 1 examined the impact of vedolizumab on concomitant medications compared with anti-TNFs in biologic naïve patients with ulcerative colitis (UC). Cohort 2 was non-comparative and observed changes in patient reported outcomes (PROs) in patients with UC and Crohn’s disease (CD) prescribed vedolizumab at any point in the treatment pathway. PROs are assessed by Short Inflammatory Bowel Disease Questionnaire (SIBDQ), IBD-Control-8 (IBD-C-8), Work Productivity and Activity Impairment (WAPI) and Rating Form of IBD Patient Concerns (RFIPC). PROs are collected prospectively at baseline, Week 14, 6 months and 12 months post-initiation via online questionnaires. The results of an interim analysis of Week 14 PROs for patients aged ≥18 years newly initiated on vedolizumab enrolled across 7 hospital sites in Cohort 2 are reported here.
Results
Sixty-one patients (21 CD, 40 UC; 51% male) were recruited, with a median age of 39.0 (IQR 32.0–55.0); and median disease duration of 9.6 years (IQR 1.7–17.2) for CD and 5.6 years (IQR 1.3–17.4) for UC. Mean total SIBDQ scores at Week 14 were: 45.2 for CD; 50.0 for UC. Scores increased by 8.5 points in CD patients and 10.2 points in UC patients in the first 14 weeks of the study. Similarly, improvements in IBD-C-8, WPAI-UC/CD sub-scores and RFIPC were observed in both CD and UC (Table 1).
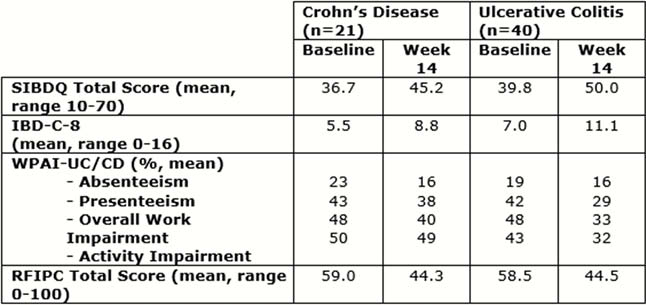 |
Conclusion
Vedolizumab treatment was associated with meaningful improvements in PROs at Week 14. Improvements were seen across all measures (SIBDQ, IBD-C-8, WPAI-CD/UC and RFIPC) and were similar between CD and UC. Further investigation of vedolizumab on PROs in the real world is required to assess impact in the longer-term, the full analysis of OCTAVO at months 6 and 12 will help to provide this.


