P714 Real-world effectiveness of vedolizumab in ulcerative colitis: Week 52 results from the Swedish multi-centre, prospective, observational SVEAH UC study
C. ERIKSSON1, S. Rundquist1, V. Lykiardopoulos2, R. Udumyan3, P. Karlén4, O. Grip5, C. Söderman6, S. Almer7, E. Hertervig8, J. Gunnarsson9, C. Malmgren10, J. Delin11, H. Strid12, M. Sjöberg13, D. Öberg14, D. Bergemalm1, H. Hjortswang2, J. Halfvarson1
1The SWIBREG SVEAH Study Group, 1Department of Gastroenterology, Faculty of Medicine and Health- Örebro University, Örebro, Sweden, 2Department of Gastroenterology, Linköping University, Linköping, Sweden, 3Clinical Epidemiology and Biostatistics, School of Medical Sciences- Örebro University, Örebro, Sweden, 4Department of Internal Medicine, Danderyd Hospital, Stockholm, Sweden, 5Department of Gastroenterology, Skåne University Hospital, Malmö, Sweden, 6Department of Internal Medicine, St Göran Hospital, Stockholm, Sweden, 7IBD-Unit-Gastroenterology, Karolinska University Hospital, Stockholm, Sweden, 8Department of Gastroenterology, Skåne University Hospital, Lund, Sweden, 9Department of Internal Medicine, Kungälv Hospital, Kungälv, Sweden, 10Takeda Pharma AB, Takeda, Stockholm, Sweden, 11Department of Gastroenterology, Ersta Hospital, Stockholm, Sweden, 12Department of Internal Medicine, Södra Älvs.borgs Hospital, Borås, Sweden, 13Department of Internal Medicine, Skaraborgs Hospital, Lidköping, Sweden, 14Department of Internal Medicine, Sunderby Hospital, Sunderbyn, Sweden
Background
Clinical trials may not readily reflect clinical practice. We aimed to assess the clinical effectiveness of vedolizumab in a real-world cohort of patients with ulcerative colitis (UC).
Methods
This is a prospective, observational, multi-centre cohort study. Eligible patients had active UC confirmed by a Mayo endoscopic subscore ≥2 at initiation of vedolizumab and had started treatment from 1/6/2015. Exclusion criteria included concurrent participation in a clinical trial in which UC treatment is dictated and contraindications to vedolizumab. All patients provided a written consent. Data on clinical characteristics, treatment patterns, disease activity and the short health scale were recorded at baseline and prospectively, using an electronic Case Record Form, integrated with the Swedish National Quality Registry for IBD (SWIBREG). The primary outcomes were A) clinical response, defined as a decrease in partial Mayo score of ≥2 and a reduction of ≥25 % from baseline, with a decrease ≥1 on the rectal bleeding score or an absolute rectal bleeding score of 0 or 1, at week 12 and B) clinical remission, defined as a partial Mayo score <2, at week 52. Continuous data are presented as median (interquartile range). Differences between baseline and follow-up visits were assessed by the Wilcoxon-signed rank test.
Results
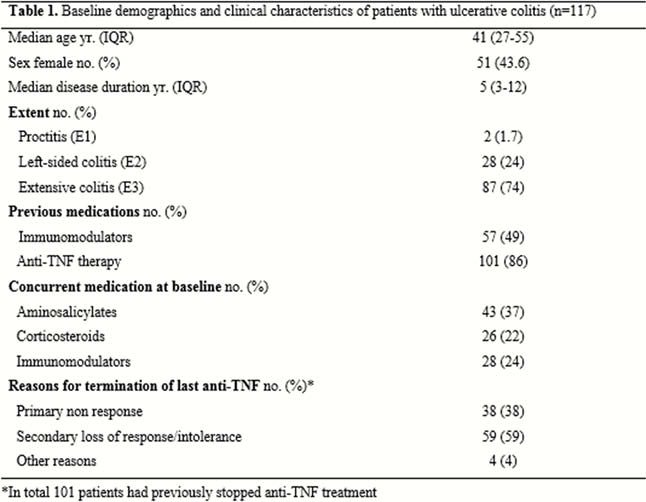
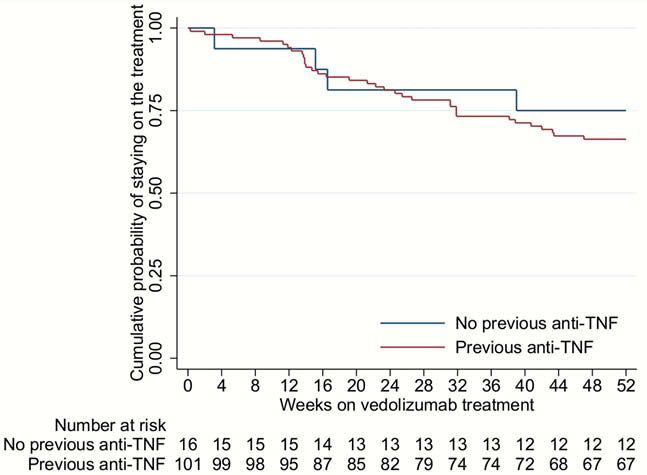
In total, 117 eligible UC patients were included during the study period 1/6/2015 to 2/6/2018. Clinical and demographical characteristics of patients are shown in Table 1; 101/117 (86%) patients had failed prior anti-TNF therapy. The drug persistence rate was 106/117 (91%) at 12 weeks and 79/117 (68%) at 52 weeks. The clinical response rate at 12 weeks was 59/117 (50%) and the clinical remission rate at 52 weeks was 57/117 (49%). Altogether, 35/117 (30%) had an endoscopic Mayo score ≤1 at 12 weeks and 41/117 (35%) at 52 weeks. However, data on endoscopy were not available for 42 vedolizumab treated patients at 12 weeks and 30 at 52 weeks. Among patients who continued vedolizumab, the median partial Mayo score decreased from 4 (3–5) at baseline to 1 (0–2) at 52 weeks (
Conclusion
Vedolizumab treated patients with UC represented a treatment-refractory group. Long-term (52 weeks) effectiveness of vedolizumab can be achieved, in terms of clinical- and inflammatory activity as well as in quality of life. The study was financially supported by Takeda.


