P722 Upadacitinib was not associated with anaemia in patients with moderately to severely active ulcerative colitis: Results from the U-ACHIEVE study
G. D’Haens1, X. Hebuterne2, P.D.R. Higgins3, E.V. Loftus- Jr.4, W. Zhou5, A.P. Lacerda5, W. Xie6, J. Liu6, S. Danese7
1Department of Gastroenterology and Hepatology- C2-112- Inflammatory Bowel Disease Center, Academic Medical Center AMC University of Amsterdam, Amsterdam, The Netherlands, 2Université de Nice-Sophia Antipolis- Hôpital de l’Archet, Faculté de Médecine, Nice, France, 3Department of Medicine- Division of Gastroenterology, University of Michigan, Ann Arbor, USA, 4Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, USA, 5AbbVie Inc., Global Pharmaceutical Research and Development, North Chicago, USA, 6AbbVie Inc., Data and Statistical Sciences, North Chicago, USA, 7Humanitas Clinico Research Centre, IBD Centre, Milan, Italy
Background
Anaemia is a frequent complication in patients with Crohn’s disease and ulcerative colitis (UC), and patients who respond to treatment generally show improvement in anaemia. Decrease in haemoglobin (Hgb) levels and anaemia may be associated with Janus kinase (JAK) inhibitors. Upadacitinib (UPA), an oral JAK1 selective inhibitor, has demonstrated favourable dose-dependent efficacy in patients with moderately to severely active UC.1 This analysis of data from the U-ACHIEVE study addressed effects of UPA treatment on Hgb levels in patients with UC.
Methods
U-ACHIEVE (NCT02819635) was a randomised, double-blind, placebo (PBO)-controlled phase 2b clinical trial. Adults with moderately to severely active UC were randomised to receive 7.5, 15, 30, or 45 mg UPA or PBO once daily (QD) for 8 weeks. Hgb levels were measured for all groups at baseline (BL) and weeks 2, 4, 6, and 8. Mean change from BL was calculated for each group.
Results
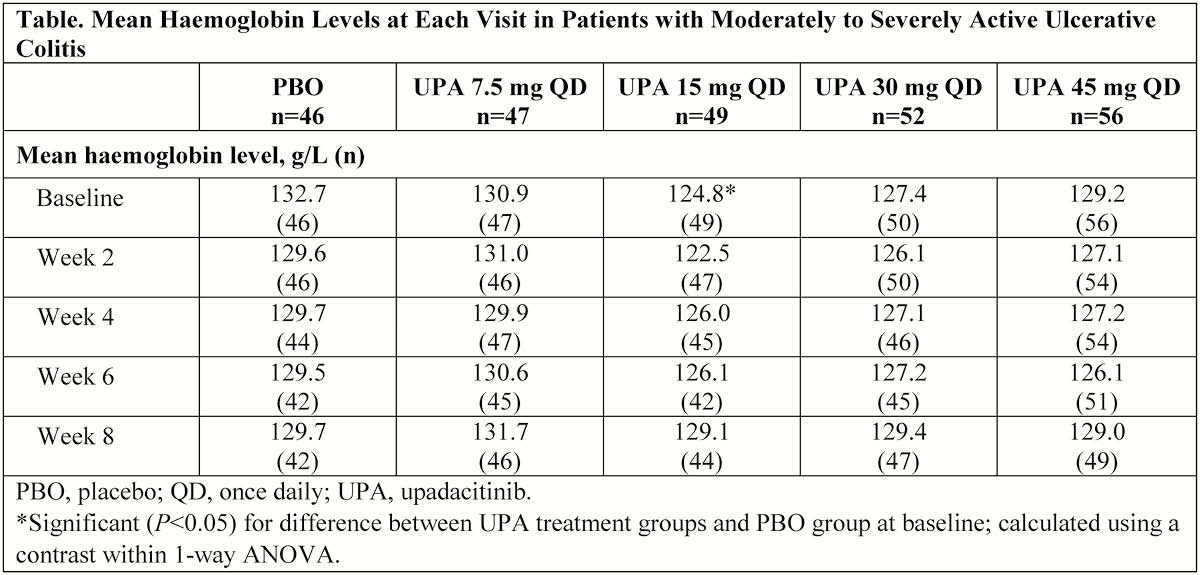
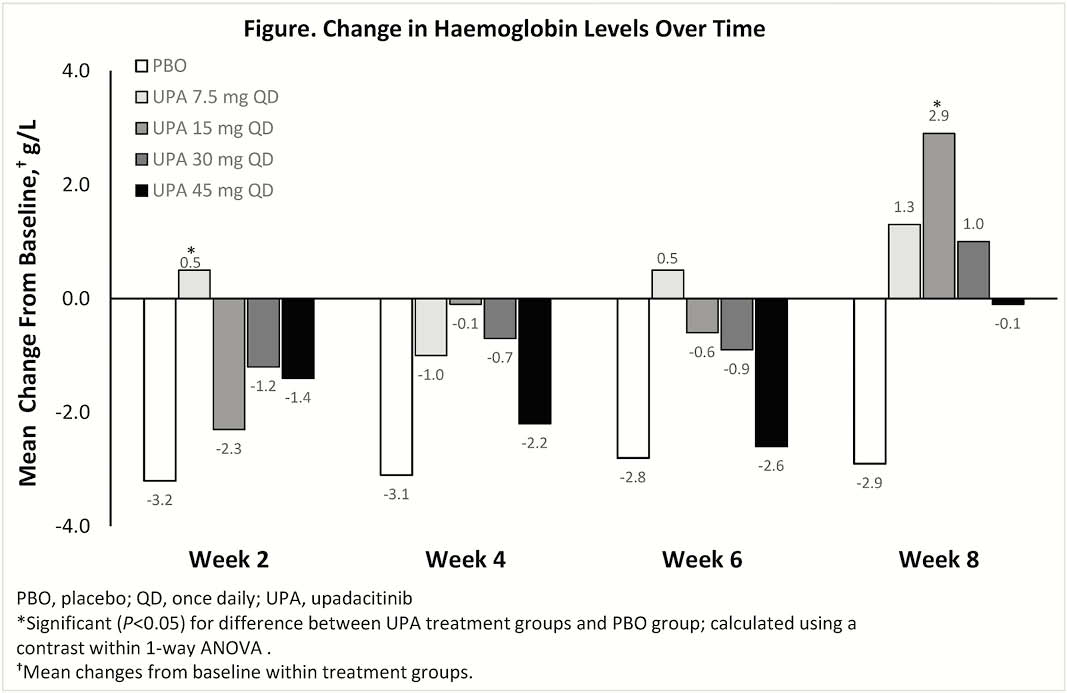
Included in the analysis were 250 patients (mean age ± SD, 42.3 ± 14.2 years; disease duration, 8.2 ± 2.5 years). At BL, most treatment groups had similar mean Hgb levels, except for the 15 mg UPA group, which had significantly lower Hgb levels vs. PBO group (Table). Increased or stable mean levels of Hgb at week 8 vs. BL were observed in most UPA treatment groups. The PBO group had the greatest decrease in Hgb vs. BL at week 8 compared with UPA treatment groups (Figure). Grade ≥2 anaemia was reported in 17.4% of patients receiving PBO and 14.4% of all patients receiving UPA (14.9%, 12.2%, 12.0%, and 17.9% of patients receiving 7.5, 15, 30, and 45 mg UPA, respectively). Grade ≥3 anaemia was reported in 8.7% of patients receiving PBO and 5% of all patients receiving UPA (6.4%, 6.1%, 6.0%, and 1.8% of patients receiving 7.5, 15, 30, and 45 mg UPA, respectively). One subject receiving 7.5 mg QD UPA had grade 4 anaemia. AE of anaemia was reported in 6.5% of patients receiving PBO and in 2.1%, 8.2%, and 3.8% of patients receiving 7.5, 15, and 30 mg UPA, respectively, but not in the 45 mg UPA group.
Conclusion
Decreased Hgb levels over time were observed more frequently in the PBO group. The maintenance of Hgb levels in patients receiving UPA was likely because of improvement of underlying UC.
Sandborne WJ, et al. United European Gastroenterology Journal. 2018;6:A74-A75.


