P723 Therapeutic thresholds for golimumab serum concentrations during induction and maintenance: Results from the GO-LEVEL study
M. Samaan1, G. Cunningham1, G. Tamilarasan1, K. Rawstron2, K. Hawash2, L. Beltran2, S. Ray1, J. Mawdsley1, S. Anderson1, J. Sanderson1, Z. Arkir2, P. Irving1
1Guy’s & St Thomas’ NHS Foundation Trust, Gastroenterology, London, UK, 2Guy’s & St Thomas’ NHS Foundation Trust, Viapath Laboratories, London, UK
Background
The exposure–response relationship associated with the use of golimumab for UC has been previously demonstrated in the PURSUIT trial programme. A significant association between serum golimumab concentrations (SGC) and favourable outcomes was observed during both induction and maintenance therapy. However, data regarding the optimal therapeutic SGC threshold is limited.
Methods
GO-LEVEL was an open-label, phase IV, investigator initiated study (NCT03124121) which included a prospective cohort of UC patients commencing golimumab induction therapy, as well as a cross-sectional cohort receiving maintenance treatment (>18 weeks from initiation).
Patients commencing induction therapy all had disease activity objectively confirmed and were evaluated at weeks 6, 10 and 14. Patients receiving maintenance therapy were recruited either at the point of flare, or during stable remission. Clinical disease activity was evaluated using SCCAI and PRO2, and biochemical activity using FC and CRP. Combined clinical-biochemical remission was defined as SCCAI<3 as well as FC<250ug/g. SGC and anti-golimumab antibodies (AGA) were measured using a drug-sensitive ELISA (LISATRACKER, Theradiag). Fishers exact or Mann–Whitney
Results
Thirty-nine patients were included in the induction cohort, of whom 15 (38%) achieved combined remission at week 6. The median SGC of those in combined remission was significantly higher than those who were not (5.0 vs. 3.1 μg/ml, respectively,
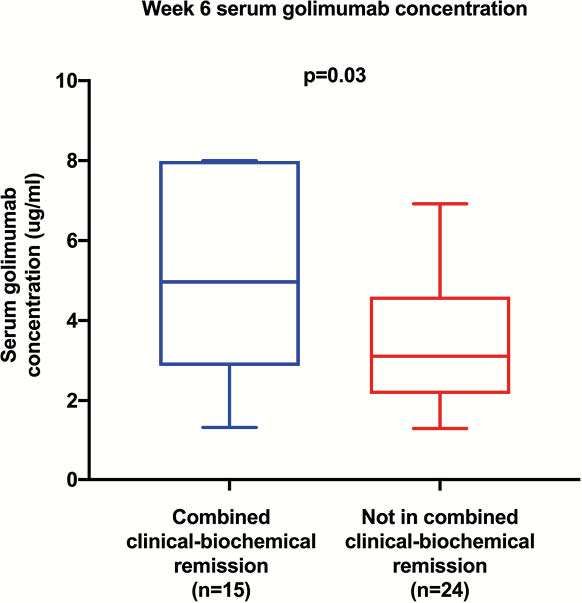
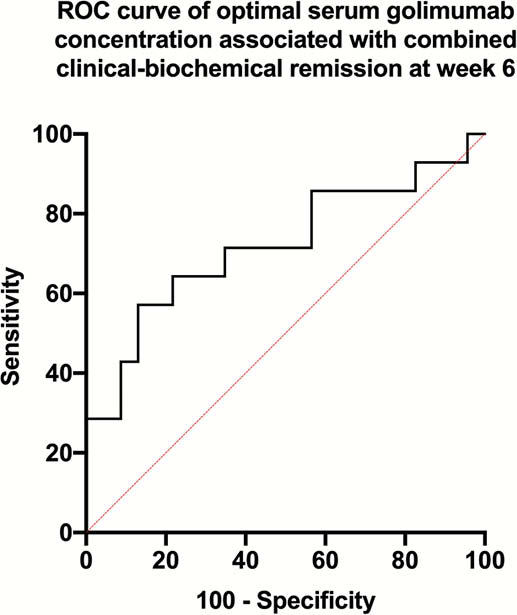
Sixty-four patients were included in the maintenance cohort; 32 (50%) were in combined remission, 32 were not. The median SGC of those in combined remission was significantly higher (3.0 vs. 2.1 μg/ml, respectively,
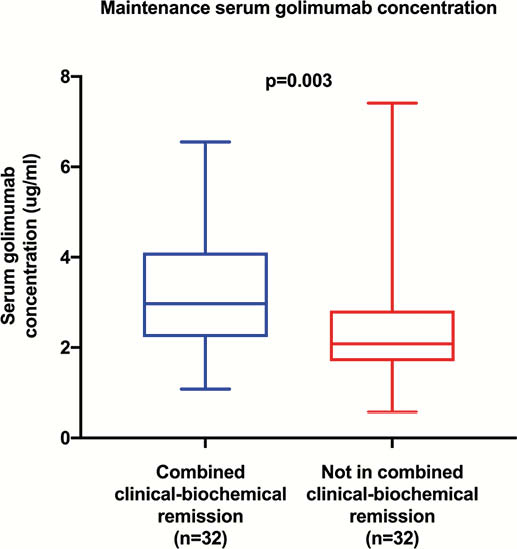
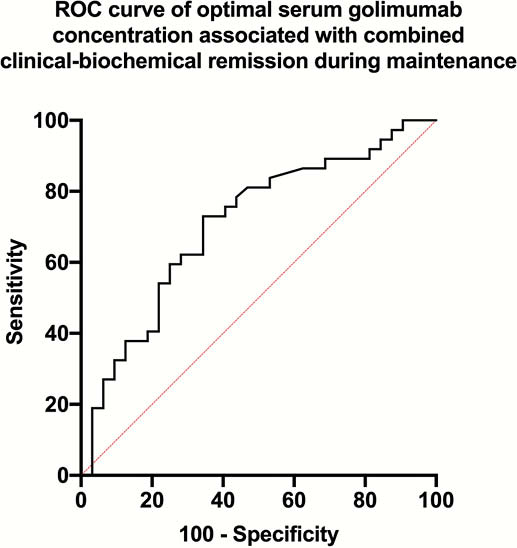
No AGA were detected in either cohort.
Conclusion
GO-LEVEL offers further evidence of the exposure-response relationship with golimumab. Clinicians may consider using therapeutic drug monitoring to optimise golimumab dosing aiming to achieve our suggested SGC therapeutic thresholds of 3.8 μg/ml at week 6 and 2.4 μg/ml during maintenance.


