P729 The SAPPHIRE registry: Safety of immunosuppression in a prospective cohort of inflammatory bowel disease patients with a HIstoRy of CancEr
J. Axelrad1, J.F. Colombel2, E. Scherl3, D. Lukin3, S. Chang1, L. Chen1, J. Kwah4, A. Swaminath5, K. Sultan5, G. Lawlor6, C. Villagra2, J. Galanko7, V. Sharpless7, S. Itzkowitz2, New York Crohn’s and Colitis Organization (NYCCO)
1Gastroenterology, New York University School of Medicine, New York, USA, 2Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, USA, 3Gastroenterology, Weill Medical College of Cornell University, New York, USA, 4Gastroenterology, Albert Einstein College of Medicine, New York, USA, 5Gastroenterology, Hofstra Northwell School of Medicine, New York, USA, 6Columbia University Medical Center, Digestive and Liver Diseases, New York, USA, 7Gastroenterology, University of North Carolina School of Medicine, Chapel Hill, USA
Background
Previous retrospective studies have demonstrated that patients with inflammatory bowel disease (IBD) exposed to anti-TNF and/or immunomodulators (IMM) following a diagnosis of cancer were not at an increased risk of new or recurrent cancer compared with those unexposed to these agents. Prospective studies are lacking and little is known about cancer risk with ustekinumab and vedolizumab. The SAPPHIRE Registry was developed to prospectively examine whether patients with IBD and a history of cancer subsequently exposed to immunosuppressive IBD therapies are at greater risk of new or recurrent cancer compared with those not exposed to immunosuppression.
Methods
We are longitudinally following patients with IBD and a histologically confirmed first cancer within the last five years from 8 centres affiliated with the New York Crohn’s and Colitis Organization (NYCCO) between 2017 and 2019. Patients receiving chemotherapy or radiation at enrolment, a first cancer more than 5 years prior to study entry, or recurrent cancer within the last 5 years are excluded. Cancers are categorised into luminal gastrointestinal (GI), hematologic, dermatologic, and other solid malignancies. Our primary outcome is the development of new or recurrent cancer stratified by immunosuppression exposure.
Results
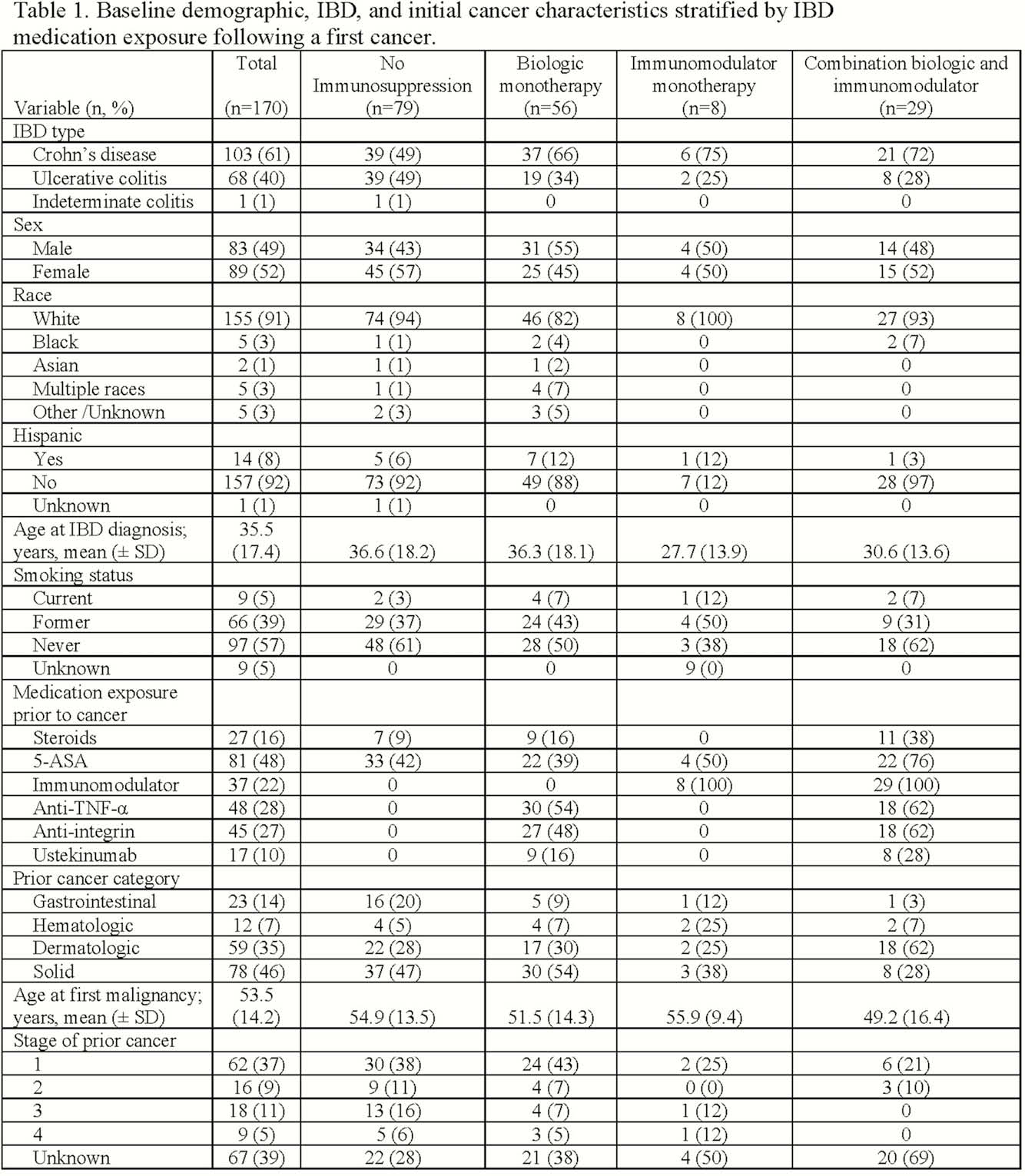
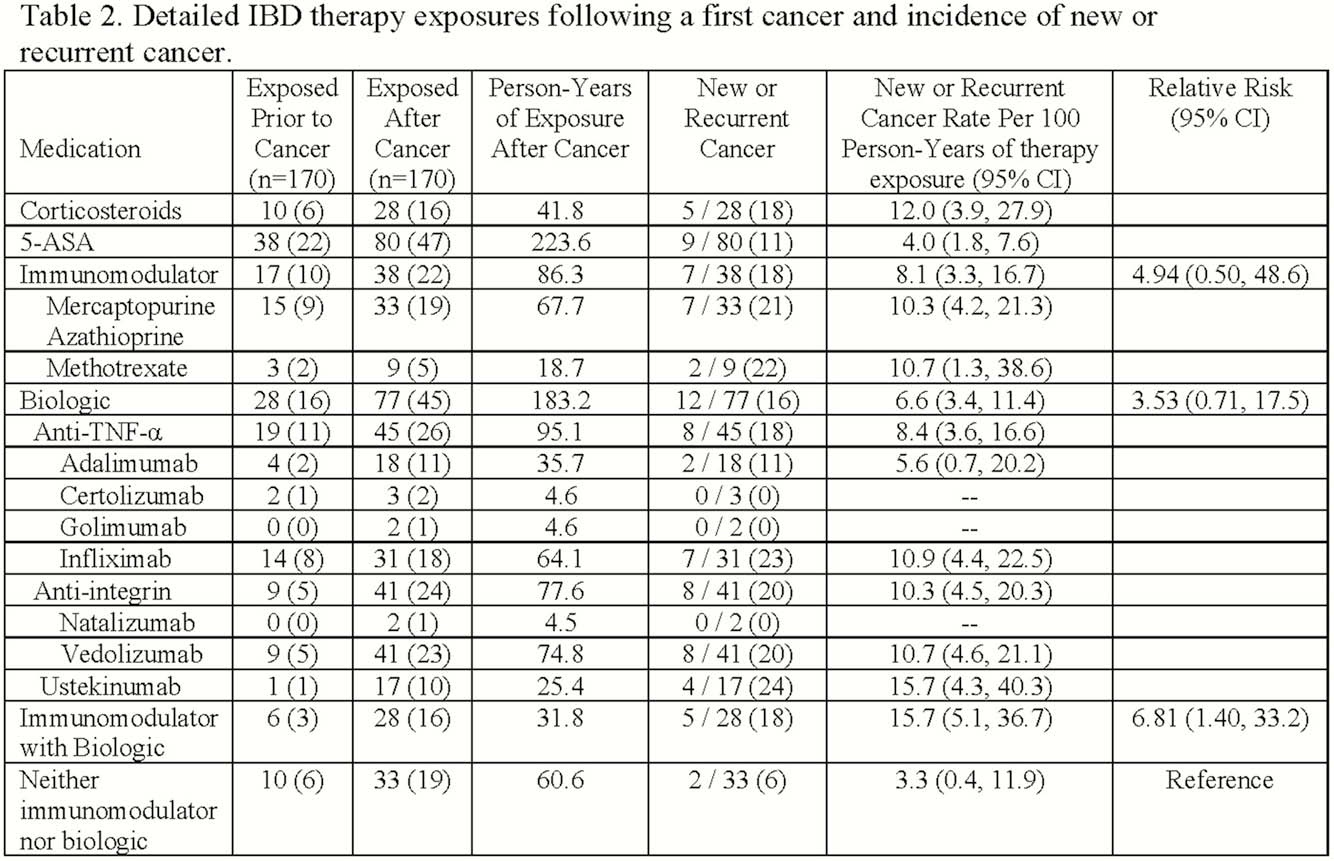
We identified 170 patients with IBD and a history of a first cancer within the last 5 years (Table 1). The average age at IBD diagnosis was 36 years; average age at cancer diagnosis was 54 years. Patients were 49% male, 91% white, and 39% former smokers. Prior cancers were solid (78; 46%), GI (23; 14%), dermatologic (59; 35%), and hematologic (12; 7%) malignancies. Following a diagnosis of cancer, patients were exposed to IMM (38; 22%), anti-TNF (45; 26%), vedolizumab (41; 23%), ustekinumab (17; 10%), or no immunosuppression (33; 19%; Table 2). During follow-up, 13 (8%) patients developed 14 subsequent cancers (6 new, 8 recurrent) comprising 6 (43%) solid, 1 (7%) gastrointestinal, and 7 (50%) dermatologic malignancies. Compared to patients not exposed to immunosuppression, exposure to an IMM (RR 4.94, 95% CI 0.50, 48.6) or a biologic (RR 3.53, 95% CI 0.71, 17.5) was not associated with an increased risk of new or recurrent cancer. However, exposure to combination therapy with an IMM and a biologic (RR 6.81, 95% CI 1.40, 33.2) was associated with an increased risk in this small sample.
Conclusion
In this ongoing prospective study, exposure to immunosuppressive IBD monotherapies in patients with IBD and a recent history of cancer has so far conferred no increased risk of new or recurrent cancer. Continued enrolment will enable a more refined estimate of the safety of combination therapies.


