P731 Effect on direct and indirect costs of switching Inflammatory Bowel Disease patients from intravenous to subcutaneous infliximab (CT-P13)
Carbery, I.(1)*;Broglio, G.(2);Clark, T.(1);Greer, D.(1);Alakkari, A.(1);Selinger, C.P.(1);
(1)Leeds Teaching Hospitals Trust, Department of Gastroenterology, Leeds, United Kingdom;(2)IRCCS Fondazione Policlinico San Matteo, Internal Medicine, Pavia, Italy;
Background
Infliximab has transformed the management of Inflammatory Bowel Disease (IBD) however it has a significant cost burden. Additionally, demand on our IBD day case infusion unit has tripled in the last 7 years leading to long delays in treatment initiation. Switching patients from intravenous (IV) to subcutaneous (SC) infliximab reduces the demand on infusion units and offers patients more flexibility. We aim to compare the real-life direct (drug price and administration costs) and indirect costs (travel costs and time lost to work for patients) associated with switching patients from IV to SC CT-P13, an infliximab biosimilar, in a tertiary UK IBD centre.
Methods
We analysed data from all adult IBD patients at Leeds Teaching Hospitals Trust who had been identified as eligible to switch from IV to SC CT-P13. All patients were on a standard dosing regimen (5mg/kg 8 weekly) and were invited to switch via a letter from the IBD lead clinician. Patients were classified as IV or SC based on their decision to switch within 3 months. Figure one demonstrates how direct and indirect costs were calculated. We then compared the calculated annual costs by intention-to-treat (i.e. if all patients had switched from IV to SC) and as-treated (comparing all on IV pre-switch to some on IV and some SC post switch).
Results
169 patients were identified as eligible to switch to SC CT-P13, 98 (58%) switched within 3 months. One patient was excluded as they had subsequently moved out of area. Prior to switching, total annual IV cost for 168 patients was calculated as £689,507.04 (direct = £653,671.20, indirect = £35,835.84). In the as-treated analysis after the switch, IV cost for 70 patients was £285,490.02 (direct = £272,363, indirect = £13,127.02) and SC cost for 98 patients was £389,432.81 (direct = £382,200, indirect = £7,232.81) giving a total annual cost for 168 patients of £674,922.83. In this analysis, after a proportion of patients switched to SC CT-P13, annual direct costs to the health service were £891.80 more per year, however indirect costs or cost to the patient were lower resulting in a lower total spend. See figure 2 for a breakdown of direct and indirect costs.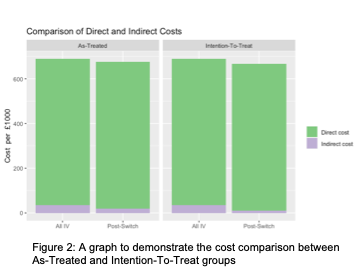
Conclusion
Our real-world analysis demonstrates that a switch from IV to SC CT-P13 is broadly cost neutral to the health service. Whilst the SC option has higher drug costs, a switch to SC allows for more efficient running of IV induction therapies by reducing demand on the infusion unit and reduces costs to the patient in terms of travel, parking and potential loss of working hours.


