P737 Intensification with intravenous ustekinumab in refractory Crohn´s disease
Arroyo Argüelles, J.M.(1);Suárez Ferrer, C.(2)*;Rueda García, J.L.(2);Martín-Arranz, E.(2);Poza Cordón, J.(2);Sánchez-Azofra, M.(2);García-Ramírez, L.(3);Martin-Arranz, M.D.(4);
(1)Hospital Universitario de Jaén, Gastroenterology Department. Hospital Universitario de Jaén- España., Jaén, Spain;(2)Grupo de enfermedades inmunomediadas gastrointestinales y otras patologías digestivas. Instituto de Investigación Sanitaria del Hospital Universitario La Paz – IdiPAZ- Madrid- España., Gastroenterology Department. Hospital Universitario La Paz- Madrid- Spain, Madrid, Spain;(3)Grupo de enfermedades inmunomediadas gastrointestinales y otras patologías digestivas. Instituto de Investigación Sanitaria del Hospital Universitario La Paz – IdiPAZ- Madrid- España., Fundación para la Investigación Biomédica- Hospital Universitario La Paz- Madrid- Spain, Madrid, Spain;(4)Grupo de enfermedades inmunomediadas gastrointestinales y otras patologías digestivas. Instituto de Investigación Sanitaria del Hospital Universitario La Paz – IdiPAZ- Madrid- España., Gastroenterology Department. Hospital Universitario La Paz- Madrid- Spain. Faculty of Medicine. Universidad Autónoma de Madrid, Madrid, Spain;
Background
There is little scientific evidence available about the results in terms of clinical and biochemical response after intensification with intravenous (IV) ustekinumab (UST) as a therapeutic strategy to be used in patients with refractory Crohn's disease (CD) who are receiving subcutaneous (SC) treatment with said drug.
Methods
Patients with CD in stable follow-up at Hospital Universitario La Paz (Madrid), who were undergoing intensified treatment with IV UST, were recruited. The objective of the study was to assess the clinical and analytical response and drug levels 12 weeks after the change from subcutaneous to intravenous UST.
Results
27 patients were retrospectively included, all of them with previous treatment with UST SC. In Table 1 baseline characteristics are collected.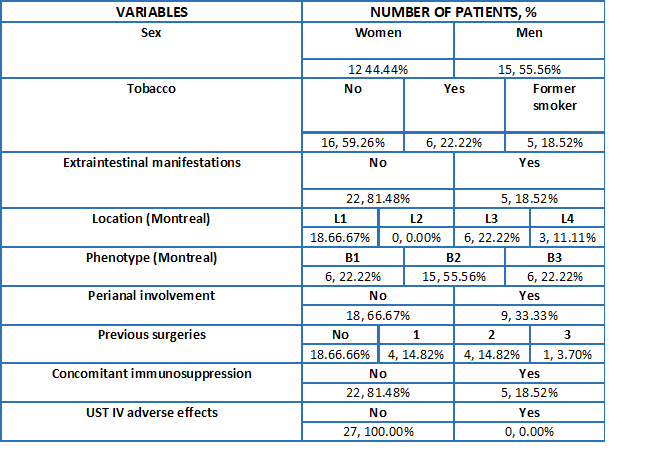
Out of the 27 patients included, 4/27 were under treatment with UST in first line, 10/27 in second line, 9/27 in third line and 4/27 had failed to three biologics.
The reason for intensification with IV UST was the lack of response to treatment in the 27 patients, 7 of them (25.93%) being found in the context of post-surgical recurrence. On the other hand, 10 patients (37.03%) were already under treatment with intensified SC UST, while the remaining 17 patients (62.96%) were under treatment with a standard schedule of SC UST every 8 weeks.
At the baseline visit, prior to the change to IV UST, differences in levels were observed between intensified and non-intensified patients (7216 vs 2842, p=0,00005). However, no significant differences were appreciated between these two groups 12 weeks after IV intensification (7949 vs 7937; p=0,99).
In patients with intensified UST SC was observed a decrease in fecal calprotectin 12 weeks after starting IV intensification, going from a mean of 1463 to 751, although the differences were not significant (p=0,14).
In Figure 1 is shown improvement in fecal calprotectin levels and in Figure 2 improvement in UST levels in blood is reflected, both parameters quantified 12 weeks after intravenous intensification of the drug.

In Table 2 data derived from the analysis of the clinical activity and determinations of CRP and calprotectin are collected, before performing the intensification with UST IV and 12 weeks after it.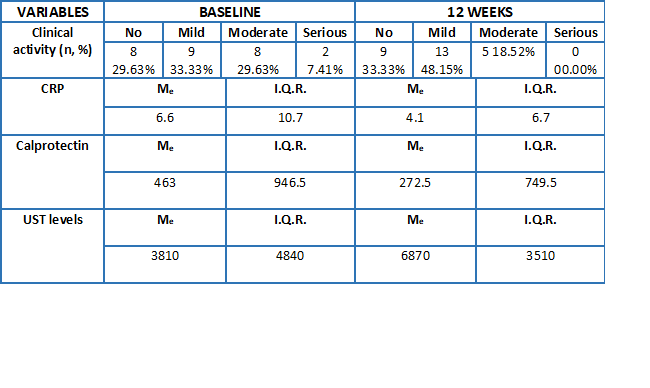
Conclusion
In our experience, change to intravenous ustekinumab in maintenance led to an improvement in the clinical and biochemical response in patients with Crohn's disease (even in patients already intensified). Likewise, an increase in drug levels was observed 12 weeks after the change from subcutaneous to intravenous treatment.


