P752 A nationwide survey of chronic enteropathy associated with SLCO2A1 gene in Japan
J. Umeno1, Y. Fuyuno1, T. Torisu1, A. Hirano1, M. Esaki2, S. Yanai3, N. Ohmiya4, T. Hisamatsu5, K. Watanabe6, N. Hosoe7, H. Ogata7, F. Hirai8, T. Hisabe9, T. Matsui9, T. Yao10, T. Kitazono1, T. Matsumoto3, CEAS Study Group
1Graduate School of Medical Sciences, Kyushu University, Department of Medicine and Clinical Science, Fukuoka, Japan, 2Saga University Hospital, Department of Endoscopic Diagnostics and Therapeutics-, Saga, Japan, 3Iwate Medical University, Division of Gastroenterology, Department of Internal Medicine, Faculty of Medicine-, Morioka, Japan, 4Fujita Health University School of Medicine, Department of Gastroenterology, Toyoake, Japan, 5Kyorin University School of Medicine, Department of Gastroenterology and Hepatology, Mitaka, Japan, 6Hyogo College of Medicine, Department of Intestinal Inflammation Research, Nishinomiya, Japan, 7Keio University School of Medicine, Center for Diagnostic and Therapeutic Endoscopy, Tokyo, Japan, 8Fukuoka University Faculty of Medicine, Department of Gastroenterology, Fukuoka, Japan, 9Fukuoka University Chikushi Hospital, Department of Gastroenterology, Chikushino, Japan, 10Sada Hospital, Gastroenterology, Fukuoka, Japan
Background
Chronic enteropathy associated with
Methods
All study participants provided written informed consent for genetic analysis. The present study was approved by the ethics committee of each institution. During the period between 2012 and 2019, 141 patients suspected of CEAS were enrolled in this study and checked to have
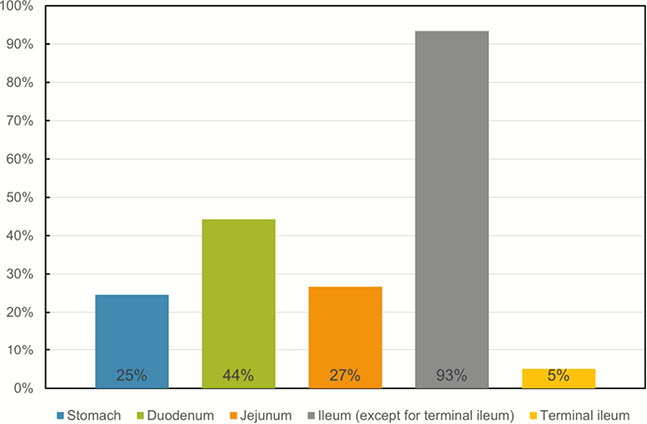
Results
We confirmed 61 CEAS patients (21 males and 40 females) and found 14 different types of recessive
Conclusion
Although CEAS frequently causes intestinal strictures like CD, its clinical features seem to be distinct from those of CD. Also, a gender difference in clinical symptoms needs to be considered, because concurrent PHO can be frequently seen especially in male patients with CEAS.


