P756 Cumulative adverse events of anti-TNF therapy in IBD patients at a large tertiary centre – a 13-year real-world experience
Majumder, S.(1,2,3);Love, M.(3);Davis, J.(3);Sharma, N.(4);Nabil Quraishi, M.(3);Cooney, R.(3);Ghosh, S.(5);Iacucci, M.(1,3,6);N Shivaji, U.(1,3,6)*;
(1)University of Birmingham, Institute of Immunology and Immunotherapy-, Birmingham, United Kingdom;(2)University of Birmingham, India Institute, Birmingham, United Kingdom;(3)University Hospitals Birmingham NHS Foundation Trust, Gastroenterology, Birmingham, United Kingdom;(4)University Hospitals Birmingham NHS Foundation Trust- Birmingham, Gastroenterology, Birmingham, United Kingdom;(5)College of Medicine and Health- University College Cork, APC Microbiome, Cork, Ireland;(6)National Institute for Health Research NIHR Birmingham Biomedical Research Centre- University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Institute of Immunology and Immunotherapy, Birmingham, United Kingdom;
Background
Anti-TNFα is the first class of biologics approved for treatment of moderate-to-severely active Inflammatory Bowel Disease (IBD). More than two decades on, anti-TNFs remain one of the most commonly used biologics for both Ulcerative Colitis (UC) and Crohn’s Disease (CD), and often as first-line therapy. Although effective, they cause several adverse events (AEs), sometimes requiring withdrawal of therapy. The aim of the study was to record cumulative AEs in IBD patients and report on real-world safety profile of this class of drugs.
Methods
All IBD patients exposed to anti-TNF therapy were identified from EMR at two centres of a large tertiary referral centre between January 2009 and June 2022. All relevant demographic and clinical data were collected for those patients with well recorded AEs and follow-up. Data on cumulative AEs directly attributed to anti-TNFs that ranged from mild reactions to serious AEs (defined as those requiring review/withdrawal of anti-TNF), were collected. Data on malignancies in this cohort was collected separately. The data were analysed and statistical analysis carried out using the IBM® SPSS® Statistics software package Version: 28.0.0.0.
Results
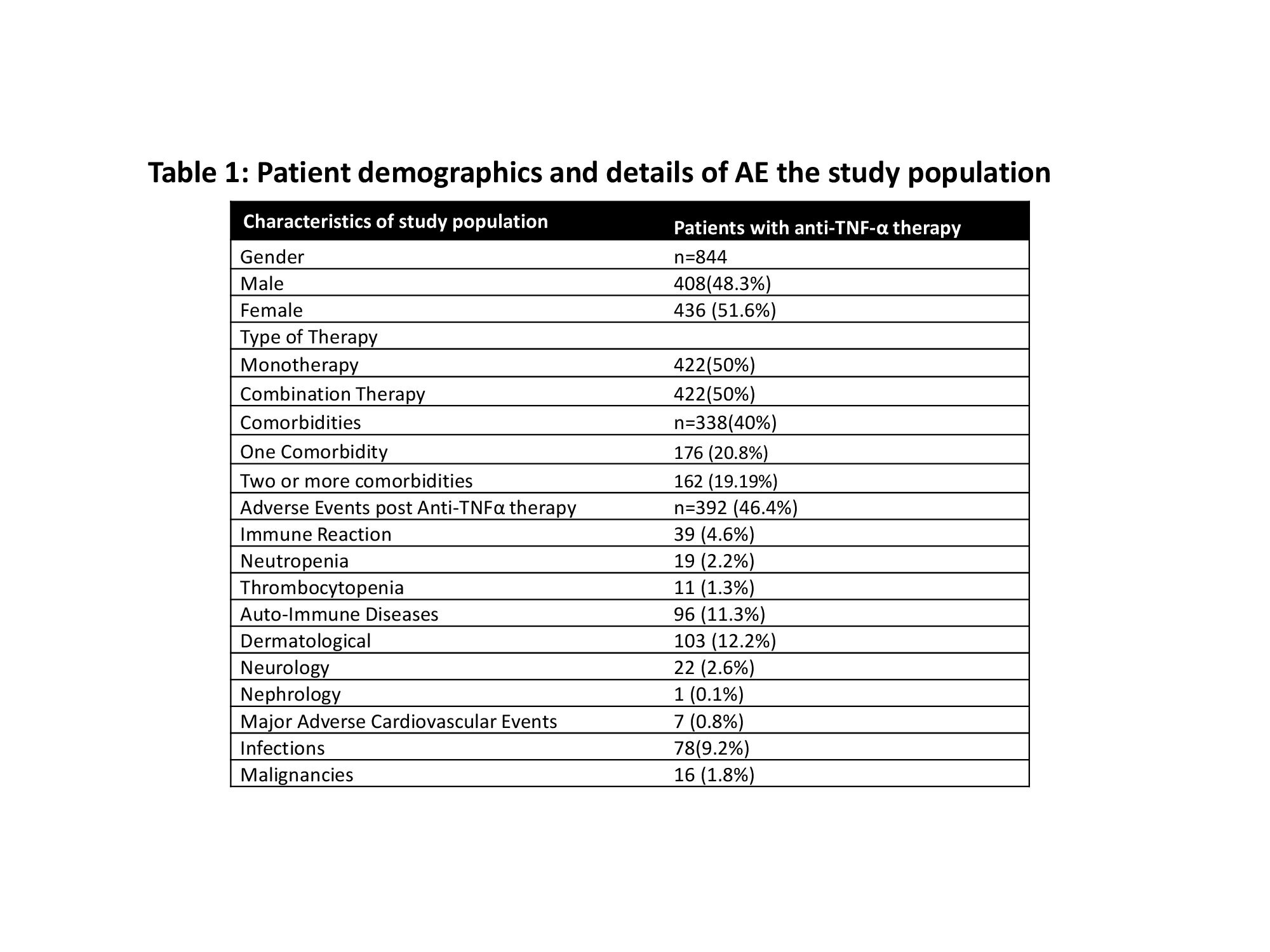
Total of 844 patients (M=408, 48%; median age 37years) were included, of which 61% were Caucasian. Majority had CD (608;72%) with mean age at diagnosis of 27y (SD 15y) and a median disease duration of 12years. About 50% of patients were on monotherapy and adalimumab was most frequently used (52%). A significant proportion of patients (44%) discontinued therapy during the follow-up period covered by the study (median duration 5years); infliximab showed best persistence rate (Fig 1). The commonest AEs were dermatological followed by auto-immune. Infections affected all age groups and bacterial infections were most common. Male sex, age groups of 18-50 years were statistically more likely to develop an AE (Table 1). 16 malignancies and 1 death were recorded in this cohort.

Conclusion
In our cohort, a significant number of AEs to anti-TNFs were noted, frequently resulting in the withdrawal of therapy. Although effective, anti-TNFs related AEs lead to poor persistence rates and contribute to increased risk of infections, particularly in >60y age group. Clinicians must be aware of the wide range of AEs due to anti-TNFs; these risks must be considered, and patients selected carefully to maximize success. With a wider choice of biologics and small molecules currently available, other options with apparently more favourable safety profiles could be considered earlier in the treatment algorithm.


