P772 Association of histological remission with long-term clinical outcomes in patients with ulcerative colitis: a multi-national retrospective study with centralized histological assessment
Jairath, V.(1,2)*;Armuzzi, A.(3,4);Pai, R.K.(5);Sun, D.(6);Li, Y.(7);Bassel, M.(8);Brett, N.R.(8);Bojic, D.(9);
(1)Western University, Department of Medicine, London, Canada;(2)Western University, Department of Epidemiology and Biostatistics, London, Canada;(3)IRCCS Humanitas Research Hospital, IBD Center, Milan, Italy;(4)Humanitas University, Department of Biomedical Sciences, Milan, Italy;(5)Mayo Clinic Arizona, Laboratory Medicine and Pathology, Scottsdale, United States;(6)Genentech- a Member of the Roche Group, Product Development Data Sciences, South San Francisco, United States;(7)F. Hoffman-La Roche AG, Product Development Data Sciences, Basel, Switzerland;(8)PPD- Part of Thermo Fisher Scientific, Real-World Evidence, Montreal, Canada;(9)F. Hoffman-La Roche AG, Product Development Medical Affairs, Basel, Switzerland;
Background
Histological remission (HR) is an achievable therapeutic target in ulcerative colitis (UC), however there is limited data on the association of HR with long-term clinical outcomes. We evaluated the association between HR and a composite outcome of colectomy, UC-related hospitalization, or intravenous (IV) corticosteroid use in patients with UC.
Methods
This was a multi-national retrospective study of adult patients with UC treated with a biologic or small molecule drug between 09/01/2005 and 06/30/2019 in Canada, Italy, Netherlands and United States. The study included biologic-naïve (bio-naïve) and biologic-experienced (bio-exp) patients. Biopsies taken during routine practice were procured and re-assessed for histological activity by blinded gastrointestinal pathologists in a central laboratory. HR was defined as the absence of neutrophilic inflammation (Nancy Histological Index ≤1 or Robarts Histological Index ≤3 plus Geboes subgrades 2B.0/3.0) at the most recent endoscopic evaluation within 3 to 9 months after treatment initiation. Cumulative risk of time-to-event outcome over 12 months from treatment initiation was evaluated using Kaplan-Meier (KM) methods and Cox proportional hazard analysis. Statistical significance was set as p-value <0.05.
Results
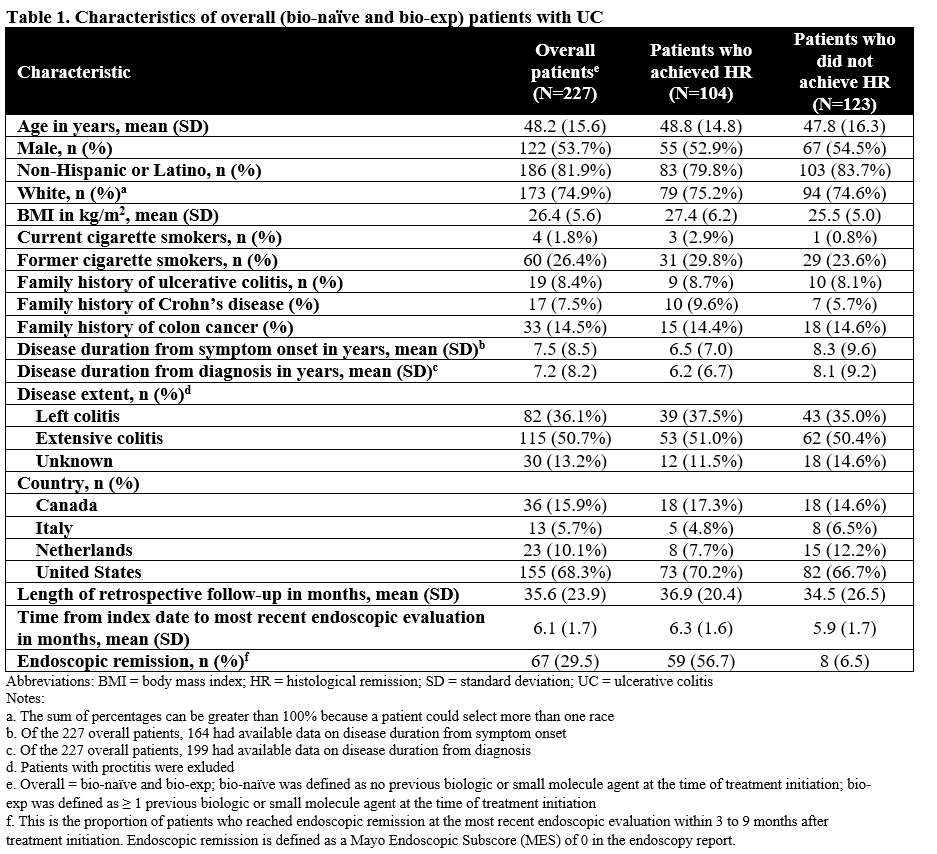
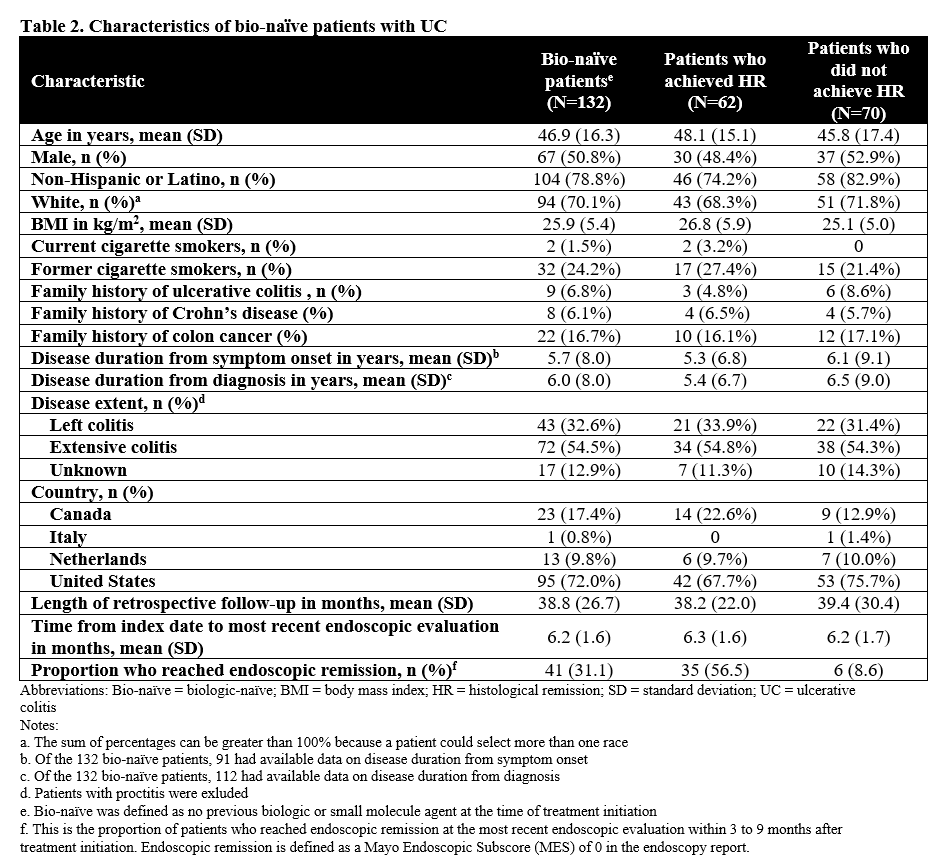
We included 227 patients with UC (bio-naïve and bio-exp) (Table 1); of whom, 132 (58%) were bio-naïve (Table 2). Of the bio-naïve patients, 62 (47%) achieved HR and 70 (53%) had histologic activity; 9 (6.8%) underwent colectomy, 16 (12.1%) had UC-related hospitalizations, and 8 (6.1%) received IV corticosteroid. Unadjusted time-to-event for the composite outcome was significantly greater in bio-naïve patients who achieved HR compared to those who did not (p=0.0023; Figure 1A). In multivariable analysis, the association of HR on the composite outcome in bio-naïve patients was not statistically significant (adjusted hazard ratio [aHR], 0.26; 95% CI, 0.02-4.42). In the overall patients (bio-naïve and bio-exp), unadjusted (p<0.001; Figure 1B) and multivariable (aHR, 0.32; 95% CI, 0.06-1.75) associations of HR and the composite outcome were similar to the bio-naïve patients.
Conclusion
We used central reading to evaluate the association of HR and clinical outcomes in patients with UC. While unadjusted KM curves showed that HR was associated with decreased risk of the composite outcome, the association was no longer significant after adjustment for covariates. The target sample size for this study was not achieved and the number of events was small, resulting in reduced statistical power. Future studies are warranted to explore the association further.