P784 Real-world use and 6-month effectiveness of vedolizumab subcutaneous in IBD patients: results of the VEDO-IBD study
Plachta-Danielzik, S.(1);Bokemeyer, B.(1,2,3)*;Efken, P.(4);Mohl, W.(5);Hoffstadt, M.(6);Krause, T.(7);Schweitzer, A.(8);Schnoy, E.(9);Ehehalt, R.(10);Trentmann, L.(11);Teich, N.(12);Atreya, R.(13);Franzenburg, S.(1);Hartmann, P.(4);Wenske, T.(1);Schreiber, S.(1,3);
(1)Competence network IBD, Study department, Kiel, Germany;(2)Interdisciplinary Crohn Colitis Centre Minden, Crohn Colitis Centre, Minden, Germany;(3)University Hospital Schleswig-Holstein- Campus Kiel, Clinic of General Internal Medicine I, Kiel, Germany;(4)Gastroenterology Practice Minden, Practice, Minden, Germany;(5)Center for Gastroenterology Saar MVZ, Practice, Saarbruecken, Germany;(6)Gastroenterology Practice Iserlohn, Practice, Iserlohn, Germany;(7)Gastroenterology Practice Kassel, Practice, Kassel, Germany;(8)Gastroenterology Practice Muenster, Practice, Muenster, Germany;(9)University Hospital Augsburg, III. medical clinic, Augsburg, Germany;(10)Gastroenterology Practice Heidelberg, Practice, Heidelberg, Germany;(11)Gastroenterology Practice Bremen, Practice, Bremen, Germany;(12)Gastroenterology Practice Leipzig, Practice, Leipzig, Germany;(13)Friedrich-Alexander-University Erlangen-Nuernberg, Medical Clinic 1, Erlangen, Germany;
Background
Since April 2020, vedolizumab (VEDO) has been available for use in IBD in intravenous (iv) and subcutaneous (sc) formulations. Due to limited data on real-world use and effectiveness, the objectives of this real-world evidence (RWE), observational, prospective study are to observe (1) the time point of conversion to VEDO sc, (2) the 6-month remission rate with VEDO sc. using data from the VEDOIBD study.
Methods
Between 2017-2020, 1200 consecutively enrolled patients with ulcerative colitis (UC) and Crohn’s disease (CD) were prospectively included in the VEDOIBD study from 45 IBD-experienced centers across Germany. Additionally, 74 VEDO-naïve patients were included with the goal of conversion to VEDO sc after an iv-induction period with VEDO (VEDO iv week 0, 2, 6). Effectiveness was measured by clinical remission (HBI ≤ 4) at month 6.
Results
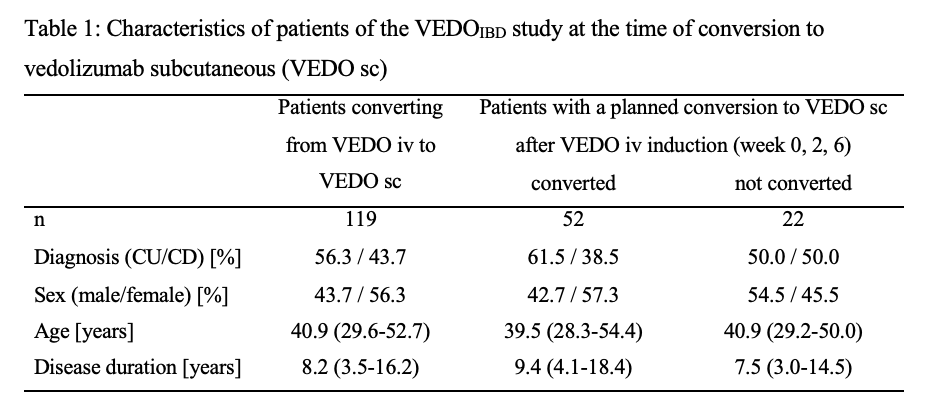
A total of 180 IBD patients with a running VEDO iv therapy were converted to VEDO sc during the entire study, with at least one 6-month visit from 119 patients (UC: n=67; CD: n=52). Characteristics are shown in Table 1. 87 IBD patients (73.1%) were still receiving VEDO sc after 6 months. 32 IBD patients (26.9%) stopped VEDO sc therapy within the first 6 months. The majority of patients returned to treatment with VEDO iv (37.9%) and the others switched to other biologics: 24.1% ustekinumab, 34.5% anti-TNF (Figure 1). At the time of conversion to sc, 92 patients (77.3%) were in remission (UC: 75%; CD: 84.6%). Of those, 88% remained in remission at 6 months (UC: 89.6%; CD: 86.4%). 45.8% of patients not in remission at the time of conversion to VEDO sc were in remission at 6 months (UC: 50%; CD: 37.5%). Of the 74 patients with planned conversion to VEDO sc, 52 patients (70.3%) received VEDO sc as planned previously: 11.5% within the first 2 weeks, 42.3% after week 6, 19.2% after week 14, and 26.8% after six months or later.
Conclusion
IBD patients with a conversion from VEDO iv to VEDO sc show high effectiveness after 6 months; conversion usually was carried out between 6 and 14 weeks. Based on these data, VEDO sc is shown to be an effective alternative to VEDO iv in RWE and should therefore be considered an important option in treatment planning and discussion with patients.