P805 Patients with Crohn’s disease treated with ustekinumab vs. vedolizumab in real-world settings
M. Chiorean Physician1, J. Jiang2, N. Candela2, G. Chen3, H. Romdhani4, D. Latremouille-Viau4, S. Shi4, R. Bungay4, T. Fan2
1Virginia Mason Medical Center, Gastroenterology, Seattle, USA, 2Takeda Development Center Americas Inc, Gastroenterology, Cambridge, USA, 3Takeda Pharmaceuticals U.S.A- Inc, Gastroenterology, Deerfield, USA, 4Analysis Group- Inc., Gastroenterology, Montreal, Canada
Background
Ustekinumab (UST) and vedolizumab (VDZ) are approved biologic therapies for moderate to severe Crohn’s disease (CD). Comparative data on real-world patient characteristics and healthcare costs for these drugs are scarce.
Methods
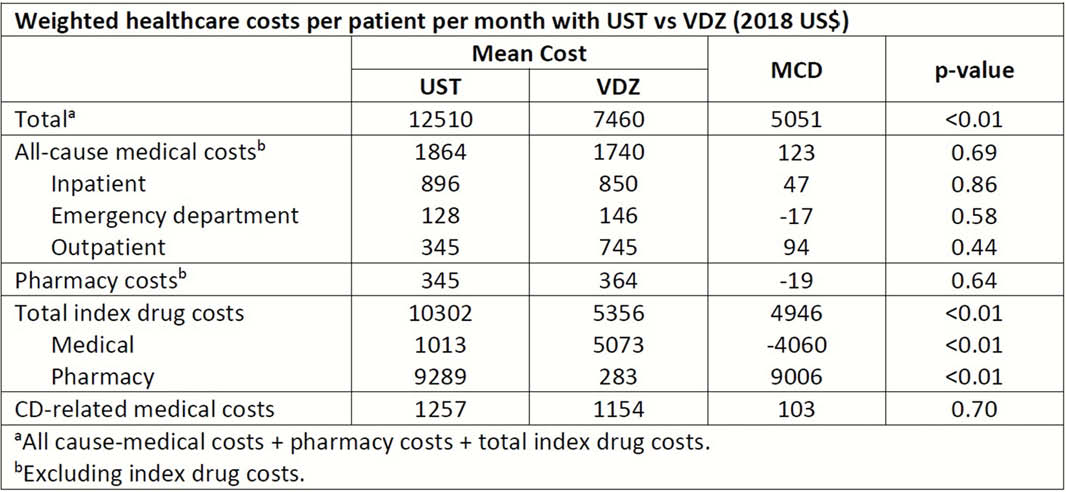
We examined healthcare costs associated with UST (healthcare common procedure coding system [HCPCS]: J3357, J3358, C9261, C9487, Q9989) and VDZ (HCPCS: C9026, J3380) in a retrospective cohort study of Truven commercial claims data (2009–2018) for adults with CD (international classification of diseases-9/10 codes: 555/K50). Eligible patients (18–89 years old) initiated UST or VDZ (index drug) on/after Sept 26, 2016, had CD as the latest relevant autoimmune disease on or before index drug initiation (index) date, ≥6 months of data available both before and after the index date, completed induction, and initiated maintenance therapy. Entropy balancing was used to address confounding factors (baseline characteristics). Primary outcome was healthcare costs assessed from a US payer perspective from index date to treatment discontinuation or end of follow-up (time on treatment). Cost were reported in 2018 US$ per patient per month and compared between treatment groups overall, and for biologic-naïve and -experienced (≥1 pre-index biologic therapy for CD) subgroups, using mean cost differences (MCD) obtained from weighted two-part models.
Results
The 599 (117 biologic-naive) UST- and 589 (172 biologic-naive) VDZ-treated patients who met eligibility criteria were similar in sex (54% and 57% female), mean age (41 ± 14 and 44 ± 14 years), time since diagnosis (42 ± 33 and 46 ± 35 months) and Charlson comorbidity index (0.4 ± 1.0 and 0.6 ± 1.1). Disease location, follow-up duration, and prior therapies and surgeries were also comparable. Characteristics were similar in biologic-naïve and -experienced patients. Mean weighted time on treatment was 11.4 and 12.1 months in UST- and VDZ-treated patients. Mean weighted total healthcare costs per patient per month was higher with UST vs. VDZ (MCD=$5051) driven by total index drug costs (MCD=$4946; Table). Cost differences were consistent in biologic-naïve and -experienced patients (total cost MCD=$4466 and $4836, both
Conclusion
Characteristics of UST- and VDZ-treated patients in real-world settings were comparable. In this population of patients receiving maintenance treatment for CD, index drug costs make UST treatment substantially more costly than VDZ. Further comparison of healthcare outcomes in patients treated with UST vs. VDZ is warranted.


