P879 Clinical significance of plasma interleukin-22 as a biomarker of inflammation in patients with Crohn’s disease and ulcerative colitis
Cabrera-Moksnes, C.(1)*;Fernandez-Llaneza, D.(2);Neisen, J.(3);Angermann, B.R.(4);Gehrmann, U.(4);Olsson, M.(5);Cairns, J.(4);Powell, N.(6);
(1)AstraZeneca, Evidence- Real World Science & Analytics, Mölndal, Sweden;(2)AstraZeneca, Evidence- Real World Science & Analytics, Cambridge, United Kingdom;(3)AstraZeneca, Translational Science & Experimental Medicine- Research and Early Development- Respiratory and Immunology R&I- BioPharmaceuticals R&D, Cambridge, United Kingdom;(4)AstraZeneca, Translational Science & Experimental Medicine- Research and Early Development- Respiratory and Immunology R&I- BioPharmaceuticals R&D, Gothenburg, Sweden;(5)AstraZeneca, BioPharma Early Biometrics and Statistical Innovation, Mölndal, Sweden;(6)Imperial College London, Faculty of Medicine, London, United Kingdom;
Background
IL-22 is a key cytokine in the pathogenesis of inflammatory bowel disease (IBD). While evidence underpinning its central role as a biomarker of pathway activation, diagnosis, severity and activity for IBD has been accumulating, little is known about its application as a biomarker in IBD in real-world practice. Here, we characterize for the first time the relationship between IL-22 plasma concentrations and the clinical characteristics of a large, real-world population of patients with IBD.
Methods
This is a descriptive analysis of patients in the Study of a Prospective Adult Research Cohort with IBD (SPARC IBD) obtained from the IBD Plexus registry of the Crohn’s & Colitis Foundation in the United States. The SPARC registry includes patients with a diagnosis of Crohn’s disease (CD) or ulcerative colitis (UC). This study evaluated baseline data collected within ± 30 days from consent date. Assessments included demographics, clinical characteristics and IL-22 plasma concentrations. Disease severity was assessed using short CD Activity Index (sCDAI; scores <150 define remission, 150–219 mild activity, ≥220 moderate-severe activity) or 6-point UC Disease Activity Index (UCDAI; scores ≤1 define remission, 2–3 mild activity, ≥4 moderate-severe activity) disease severity categories.
Results
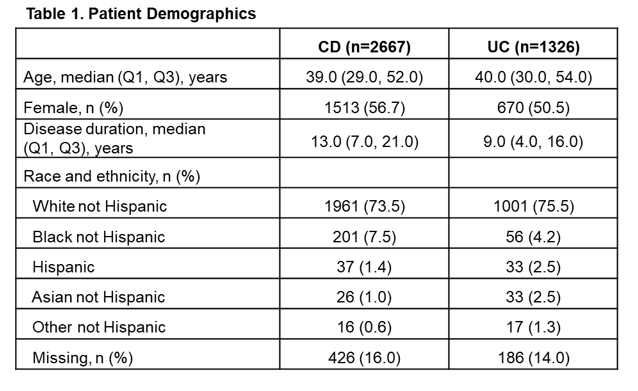
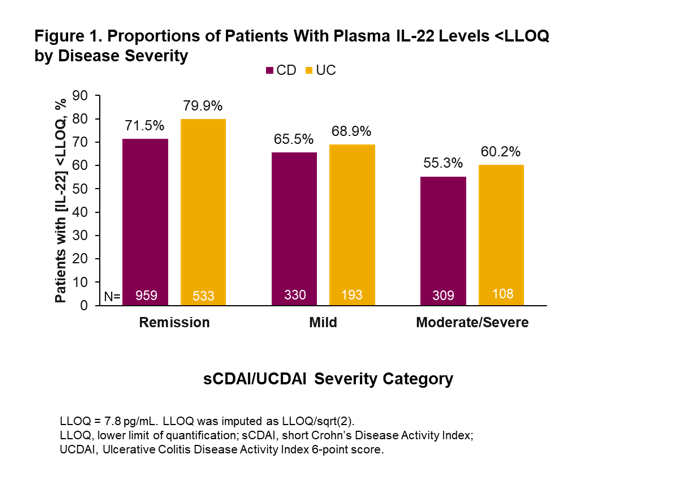
A total of 2667 patients with CD and 1326 patients with UC were eligible for inclusion in the study. For patients with CD, median age was 39 years, 57% were female and median disease duration was 13 years (Table 1). For patients with UC, median age was 40 years, 51% were female and median disease duration was 9 years (Table 1). In total, 1604 patients with CD and 836 patients with UC had valid IL-22 samples at baseline, of which 33% and 25%, respectively, were above the lower limit of quantification (LLOQ; 7.8 pg/mL). There was a trend toward fewer patients with IL-22 levels below the LLOQ among patients with high disease severity compared with those with lower disease severity both in patients with CD and those with UC (Figure 1). Additionally, there was an increase in the percentage of patients with IL-22 levels greater than the third quartile (overall Q3 = 10.8 pg/mL in CD, and = 7.9 pg/mL in UC) with increased disease severity (Figure 2). Comparison of subgroups of patients with low and medium/high IL-22 levels showed a consistent pattern with fewer patients in remission and more moderate-severe patients in the medium/high IL-22 group than in the low IL-22 group (Table 2).
Conclusion
Higher IL-22 levels tended to be associated with higher disease activity in this real-world population of IBD patients.