P888 Evaluating patient access to infliximab subcutaneous in inflammatory bowel disease
Jang, M.(1)*;Yoo, H.(1);Kwon, T.(1);
(1)Celltrion Healthcare co Ltd, Market Access, Seoul, Korea- Republic Of;
Background
Infliximab(IFX) subcutaneous(SC) was developed as a novel formulation biosimilar added to the intravenous(IV) infliximab. Infliximab is one of the most effective treatments in IBD and approval of subcutaneous formulation was expected to bring greater patient convenience, yet it had to overcome its hurdles in Health Technology Assessment (HTA) in the absent of the SC originator. This research aims to evaluate patient access to IFX SC based on time-to-reimbursement decision post market authorization in July of 2021.
Methods
The pricing database, IQVIA MIDAS® data and epidemiology data were combined to analyze patient access in 32 countries. We evaluated 1) status of the reimbursement 2) volume consumed in a country 3) average period from marketing authorisation to positive reimbursement decision 4) reason for delayed access.
Results
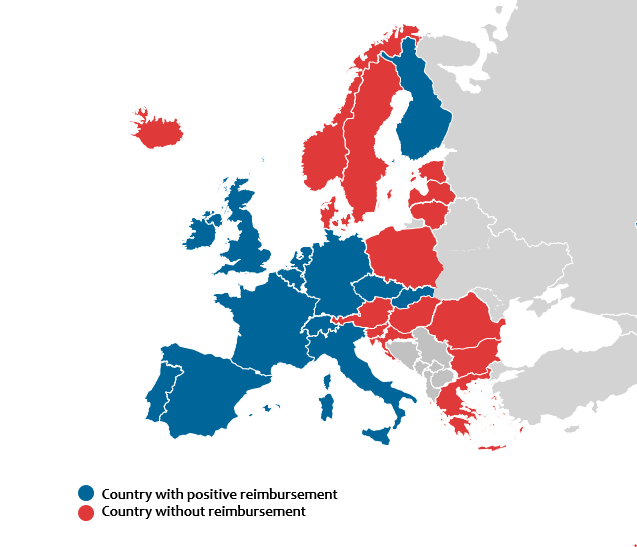
Since the market authorization, positive reimbursement decisions were made in 18 countries among 31 European countries including UK. Among them, patients who reside in 15 countries had records of utilization of IFX SC in the year of 2022. About 74.76% of IBD patients is eligible for reimbursement of CT-P13 SC among the assumed 1,023,765 IBD patients in Europe. Average time from market authorisation to positive reimbursement decision by health authorities were 9 months(2-17) in the case of IFX SC which suggests delayed reimbursement compared to biosimilars. Although most of the European countries offered shortened or simplified HTA pathways to biosimilars, most of the countries did not have clear guidance on pricing of the novel biosimilars. Several countries failed to provide IFX SC after a positive reimbursement decision because the procurement system failed to differentiate a novel biosimilar with different strength and form from the existing biosimilars in same molecule. This may have deterred the market entry; countries where adopted national tender grouped IFX SC with IFX IV(Norway, Hungary) or extended procurement basket to all available IBD treatment(Denmark and Lithuania).
Figure: Reimbursement status of infliximab subcutaneous in European countries
Conclusion
There are apparent differences in patient access to IFX SC among European countries. Biosimilars promises to bring improved patient access to biologics, however, pricing of bio-betters or bio-innovators could be convoluted that efforts are still warranted in order to bring the drug into the market within shortest possible time. Although IBD treatment options are expanding, IFX is still widely used. Adding another SC treatment may improve patient convenience and quality of life and thus, overall improved access to the drug is imperative for equitable use.