Johnson & Johnson-sponsored activities at the 20th Congress of ECCO, Berlin, Germany, 19–22 February 2025
Johnson & Johnson Satellite Symposia at ECCO 2025, Berlin, Germany
Date of preparation: March 2025 EM-177264
Introduction
Johnson & Johnson sponsored two dynamic satellite symposia at the 20th Congress of the European Crohn’s and Colitis Organisation (ECCO 2025), each diving deep into pressing topics within the field of inflammatory bowel disease (IBD).
The first symposium, tailored for Crohn’s disease (CD), was entitled “Expanding the universe in IBD: going beyond in Crohn’s disease”. The esteemed experts Prof. Silvio Danese, Prof. Iris Dotan, Prof. Mathurin Fumery and Prof. Joana Torres led engaging discussions that highlighted innovative approaches in CD management. The second symposium focused on ulcerative colitis (UC), under the title “Expanding the universe in IBD: going beyond in ulcerative colitis”, featuring insights from Prof. Raja Atreya, Dr Tim Raine and Prof. Séverine Vermeire.
If you were not able to attend these interesting and influential symposia, keep reading for a summary of the highlights, including key efficacy and safety data from studies with guselkumab and how guselkumab could shape the future landscape of IBD care.
Symposium 1: Expanding the universe in IBD: going beyond in Crohn’s disease
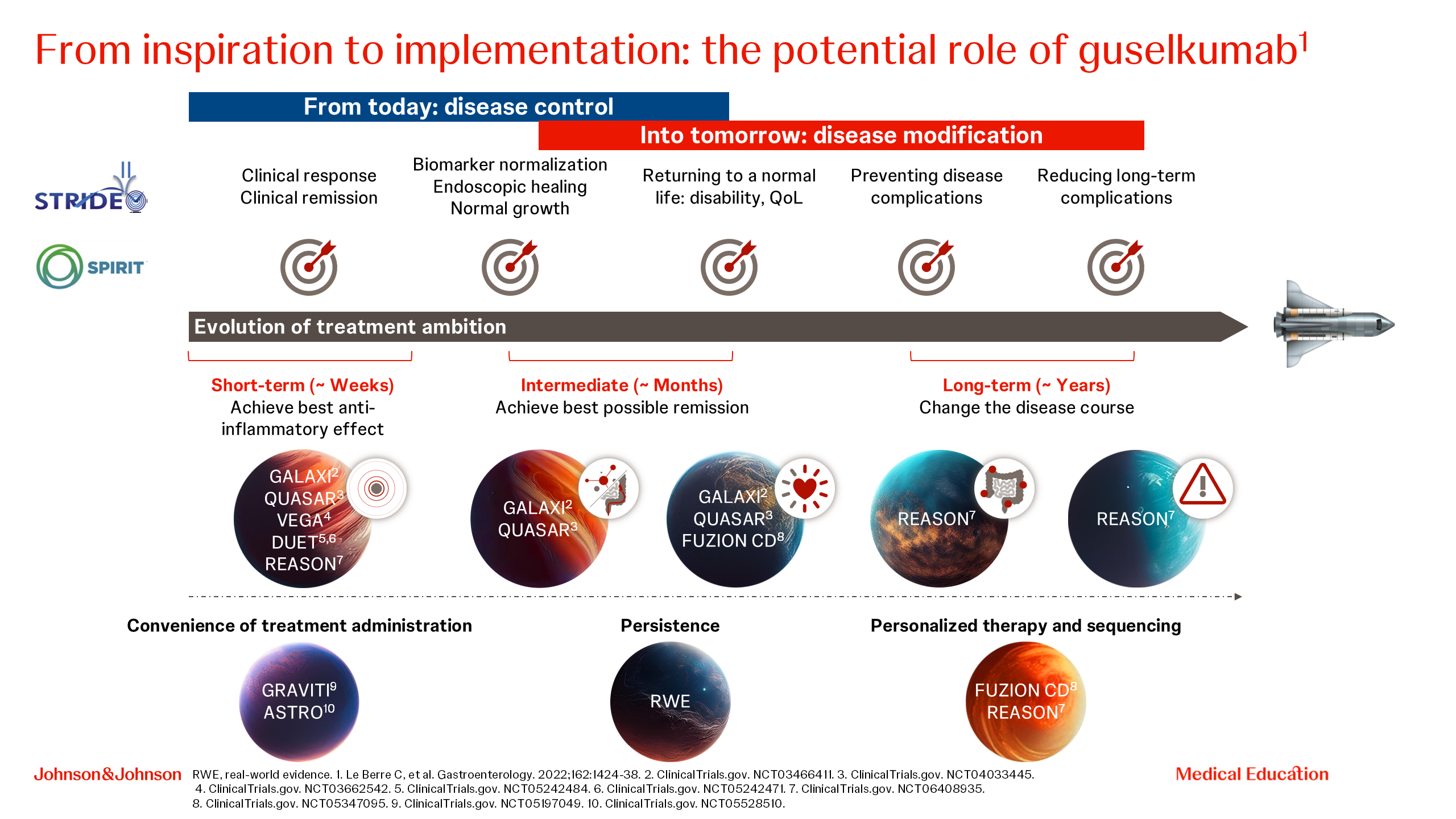
Prof. Iris Dotan opened the symposium by emphasizing the suboptimal disease control for patients with IBD with current medications, and a series of unmet needs for patients with IBD, including the lack of consideration of patient preferences in current therapy [1–6]. She then outlined how the comprehensive clinical development programme of guselkumab aims to address these unmet needs (Figure 1).
Figure 1. Guselkumab clinical development programme.
Following this, Prof. Joana Torres described the relevance of CD64-expressing myeloid cells in IBD, noting that myeloid cells that express CD64 are the primary cellular source of IL-23 in the inflamed gut [7, 8]. Then Prof. Joana Torres highlighted the dual-acting properties of guselkumab in vitro, showing that guselkumab is the only interleukin (IL)-23 inhibitor that can bind to CD64 and simultaneously capture endogenous, locally secreted IL-23 at its cellular source [9].
Moving on to the latest data from clinical trials, Prof. Mathurin Fumery and Prof. Joana Torres described the study design, patient baseline characteristics, data on short- and long-term goals, quality of life, (QoL) and safety from GALAXI and GRAVITI studies of guselkumab in CD. In these clinical trials, guselkumab has demonstrated high efficacy across short- (clinical remission at Week 12), intermediate- (quality of life, Patient-Reported Outcomes Measurement Information System-29 [PROMIS-29]) and long-term (clinical remission and endoscopic response at 1 year) goals, irrespective of previous treatment history [10–13]. Furthermore, the safety profile of guselkumab was confirmed in 3006 patients across four IBD trials and shown to be consistent with the known safety profile of guselkumab [14].
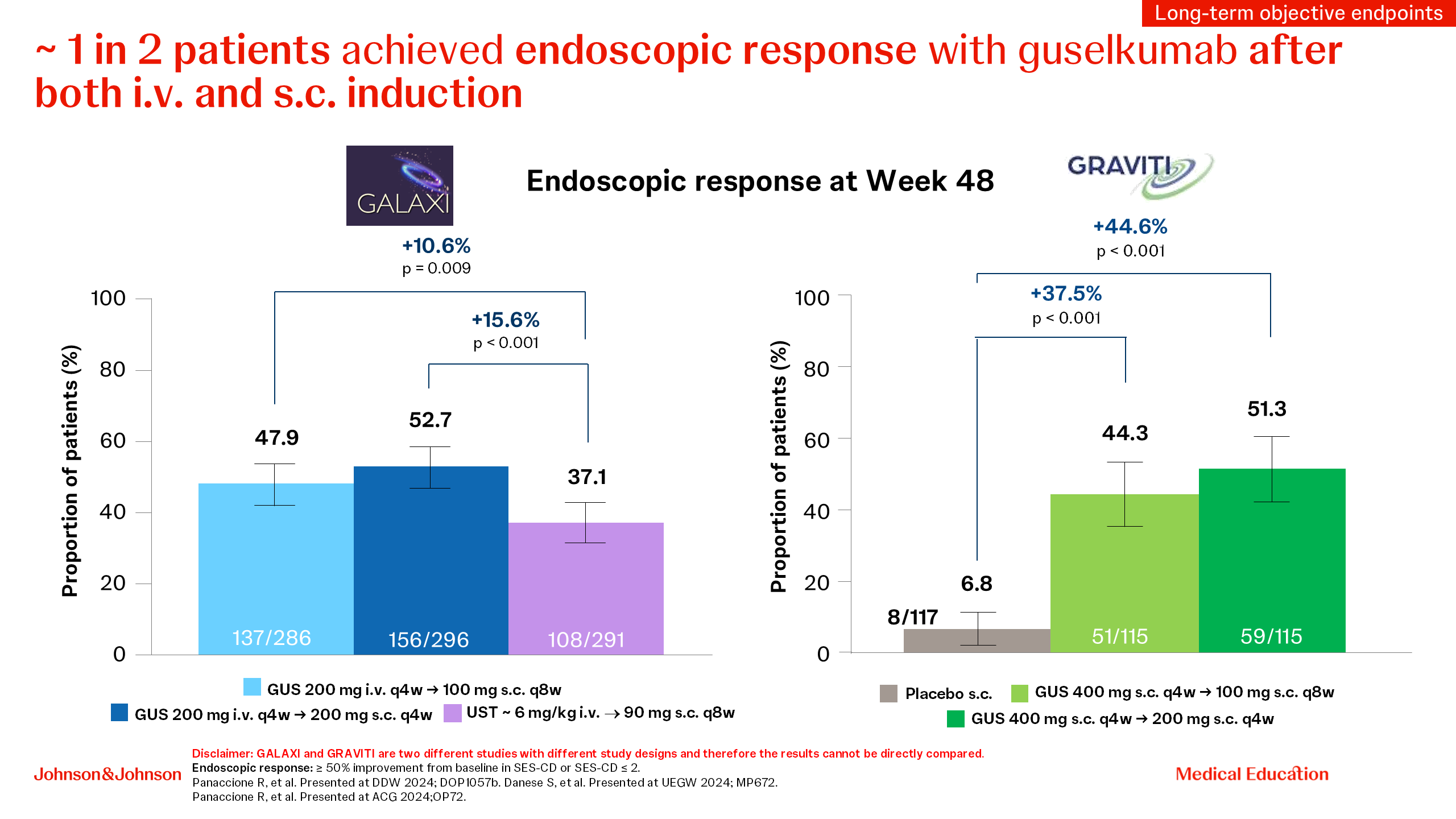
Prof. Silvio Danese then shifted from clinical to high-bar objective endpoints, outlining the value of a treat-through double-blind study design used in the GALAXI and GRAVITI trials, and explained the clinical relevance of aiming for higher treatment goals by using composite endpoints. Then he emphasized the superiority of guselkumab over ustekinumab across endoscopically driven endpoints (endoscopic response, endoscopic remission, deep remission and clinical remission plus endoscopic response) [10, 12] in the GALAXI study, with particularly high outcomes in biologic-naive patients treated early in their disease course (disease duration of ≤2 years), and also consistently observed in both biologic-naive and biologic-experienced patients [15]. When looking at the high-bar endoscopic outcomes in both GALAXI and GRAVITI studies, both intravenous (i.v.) and subcutaneous (s.c.) guselkumab induction led to approximately one in two patients treated achieving endoscopic response (Figure 2), offering flexibility to patients and physicians [10–12]. He concluded his presentation by looking into the future, mentioning the important ongoing guselkumab studies looking at fistulizing disease and transmural healing that aim to address the unmet needs in IBD [16,17].
Figure 2. Endoscopic response at Week 48 with guselkumab in the GALAXI and GRAVITI studies.
The symposium concluded with a panel discussion among all faculty members, weighing up patient perspectives and physician perspectives. In conclusion, guselkumab with its proven efficacy, well-established safety profile, flexibility of s.c. and i.v. induction and a comprehensive clinical development programme, could expand the possibilities for patients with CD.
Symposium 2: Expanding the universe in IBD: going beyond in ulcerative colitis
Prof. Raja Atreya started by describing the role of IL-23 in the pathophysiology of IBD, noting that both CD64 and IL-23 are upregulated in the inflamed gut, and overexpression of IL-23 in inflamed tissue results in impaired barrier function (and vice versa) and drives continued inflammation via innate and T cell-mediated responses [18]. He ended with the message that guselkumab is able to bind CD64 and capture IL-23 at its cellular source through its native fragment crystallizable (Fc) region, in vitro [9, 19].
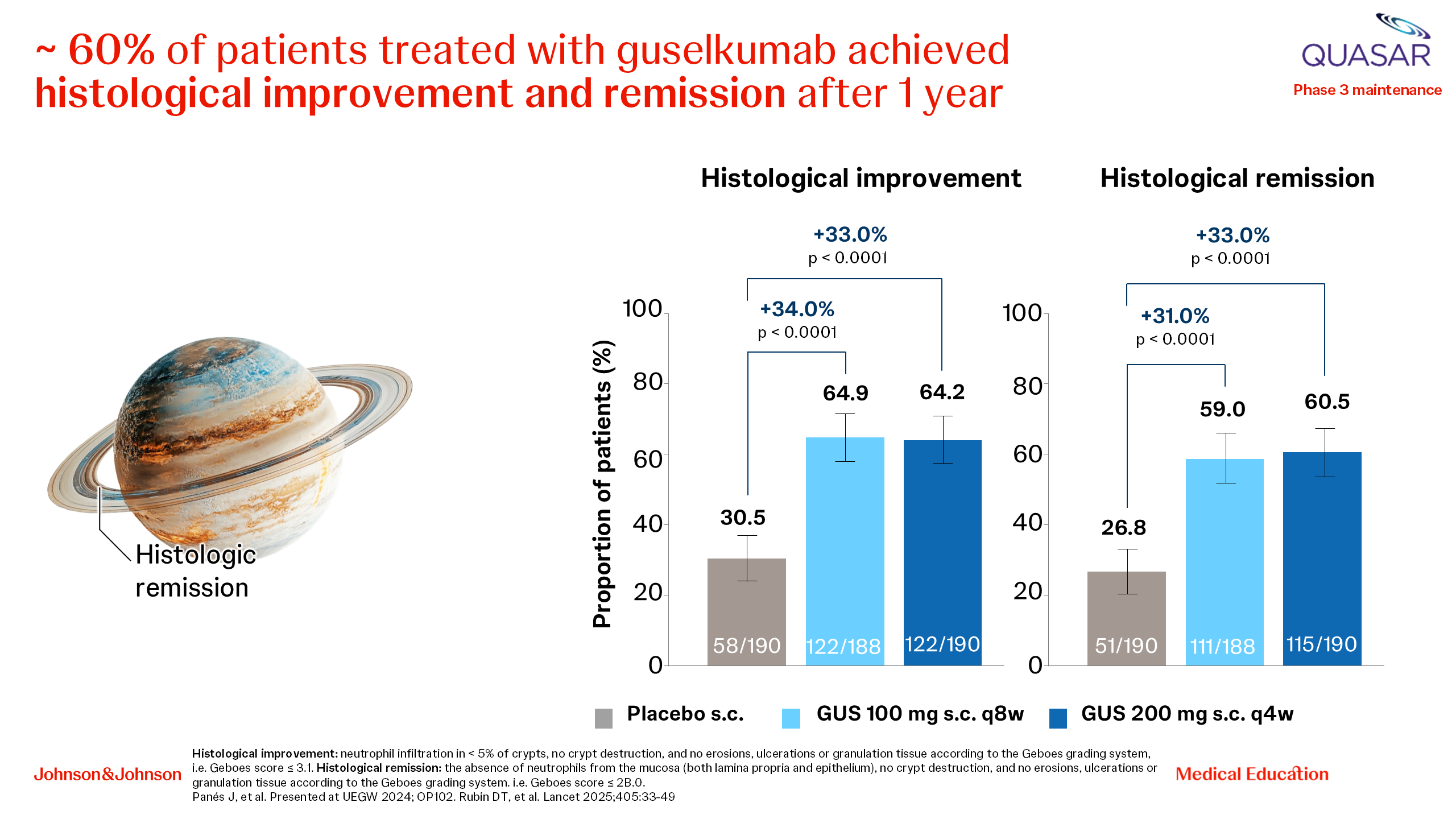
Prof. Raja Atreya and Prof. Séverine Vermeire then discussed how guselkumab could move the needle towards holistic, patient-centric and value-based care. They outlined the results from the QUASAR study with guselkumab in UC, starting with high clinical remission in the short term (Week 12) and the longer term (Week 44), the rapid symptomatic improvement with guselkumab and the high rates of clinical remission at 1 year irrespective of previous treatment history. They emphasized that 72% of patients treated with guselkumab in QUASAR maintained clinical remission through Week 44 irrespective of previous advanced therapy use and highlighted the clinically meaningful improvements compared with placebo in measures of health-related QoL. Furthermore, at Week 44, 98% of guselkumab-treated patients in clinical remission were corticosteroid-free. In terms of more objective endpoints, 35% of patients achieved endoscopic remission and 61% achieved histological remission (Figure 3) [20, 21].
Figure 3. Histological endpoints with guselkumab in the QUASAR study (UC).
Dr Tim Raine then presented the results of the ASTRO trial with guselkumab s.c. induction in UC [22], which confirmed the QUASAR results in another patient population and showed the efficacy of guselkumab s.c. induction at Week 12 across clinical and endoscopically driven endpoints. Taken together, these data show that guselkumab is the only IL-23 inhibitor to have demonstrated efficacy in clinical trials with both i.v. and s.c. induction in UC, offering flexibility and more choices for patients and physicians.
During the last session of the symposium, the faculty discussed several key issues, including taking patient preferences into account when making treatment choices, helping patients return to a normal life, what improvements in histology mean for patients and finally the safety profile of guselkumab across IBD.
To summarize, this symposium discussed the role of IL-23 and CD64-expressing myeloid cells in IBD, described the evolving treatment goals in UC and how guselkumab may help to achieve these, discussed the clinical relevance of efficacy and safety from the QUASAR and ASTRO studies with guselkumab and indicated how guselkumab may offer flexibility for patients and physicians.
References
- D'Amico F, Gomollón F, Bamias G, et al; IBD PODCAST investigators. Proportion of inflammatory bowel diseases patients with suboptimal disease control in daily clinical practice-Real-world evidence from the inflammatory bowel diseases-podcast study. United European Gastroenterol J. 2024;12(6):705-716. doi: 10.1002/ueg2.12572.
- Barberio B, Gracie DJ, Black CJ, Ford AC. Maintenance of clinical remission with biologics and small molecules in inflammatory bowel disease according to trial design: Meta-analysis. Dig Liver Dis. 2024;56(1):7-14. doi: 10.1016/j.dld.2023.06.009.
- Narula N, Wong ECL, Dulai PS, et al. Comparative Effectiveness of Biologics for Endoscopic Healing of the Ileum and Colon in Crohn's Disease. Am J Gastroenterol. 2022;117(7):1106-1117. doi: 10.14309/ajg.0000000000001795.
- Komatsu A, Toyonaga T, Sumiyoshi N, et al. Endoscopic healing is associated with a reduced risk of biologic treatment failure in patients with ulcerative colitis. Sci Rep. 2024;14(1):303. doi: 10.1038/s41598-024-51208-2.
- Xu F, Liu Y, Greenlund K, Carlson S. Trends and demographic patterns in biologic and corticosteroid prescriptions for inflammatory bowel disease: findings from electronic medical records, 2011-2020. J Investig Med. 2022;70(8):1771-1776. doi: 10.1136/jim-2022-002486.
- Al Khoury A, Balram B, Bessissow T, et al. Patient Perspectives and Expectations in Inflammatory Bowel Disease: A Systematic Review. Dig Dis Sci. 2022;67(6):1956-1974. doi: 10.1007/s10620-021-07025-y.
- Chapuy L, Bsat M, Sarkizova S, et al. Two distinct colonic CD14+ subsets characterized by single-cell RNA profiling in Crohn's disease. Mucosal Immunol. 2019;12(3):703-719. doi: 10.1038/s41385-018-0126-0.
- Chapuy L, Bsat M, Rubio M, et al. IL-12 and Mucosal CD14+ Monocyte-Like Cells Induce IL-8 in Colonic Memory CD4+ T Cells of Patients With Ulcerative Colitis but not Crohn's Disease. J Crohns Colitis. 2020;14(1):79-95. doi: 10.1093/ecco-jcc/jjz115.
- Atreya R, Allegretti JR, Abreu MT, et al. Guselkumab Binding to CD64+ IL-23–producing Myeloid Cells Enhances Potency for Neutralizing IL-23 Signaling. Presented at UEGW 2024;OP193.
- Danese S, Afzali A, Panaccione R, et al. Week 48 Efficacy of Guselkumab and Ustekinumab in Crohn’s Disease Based On Prior Response/Exposure to Biologic Therapy: Results from the GALAXI 2 & 3 Phase 3 Studies. Presented at UEGW 2024; MP672.
- Sands BE, D’Haens G, Danese S, et al. Efficacy of Guselkumab versus Placebo in Crohn’s Disease Based On Prior Response/Exposure to Biologic Therapy: Results of the GALAXI 2 & 3 Phase 3 Studies. Presented at UEGW 2024; OP042.
- Panaccione R, Hart A, Steinwurz F, et al. Efficacy and Safety of Subcutaneous Guselkumab Induction Therapy in Patients With Moderately to Severely Active Crohn’s Disease: Results Through Week 48 From the Phase 3 GRAVITI Study. Presented at ACG 2024;OP72.
- Sands BE, Danese S, Cao Q, et al. Guselkumab improves health-related quality of life as measured by PROMIS-29 in participants with moderately to severely active Crohn’s disease: Phase 3 GRAVITI study. Presented at ECCO 2025; P1149.
- Sands BE, Panaccione R, Danese S, et al. Safety of Guselkumab in Inflammatory Bowel Disease Up to 1 Year: Integrated Safety Analysis of Phase 2 and 3 Studies in Crohn’s Disease and Ulcerative Colitis. Presented at ECCO 2025; P0608.
- Afzali A, Rubin DT, Danese S, et al. Early disease efficacy of guselkumab therapy in biologic-naïve patients with moderately to severely active Crohn’s disease: Post-hoc analysis from the phase 3 GALAXI 2 & 3 studies. Presented at ECCO 2025; DOP060.
- A Study of Guselkumab in Participants With Fistulizing, Perianal Crohn's Disease (FUZION CD). ClinicalTrials.gov. NCT05347095. Available from: Study Details | A Study of Guselkumab in Participants With Fistulizing, Perianal Crohn's Disease | ClinicalTrials.gov. Accessed 11 April 2025.
- Transmural Healing and Disease-Modifying Effect of Guselkumab in Crohn's Disease Patients (REASON). ClinicalTrials.gov. NCT06408935. Available from: Study Details | Transmural Healing and Disease-Modifying Effect of Guselkumab in Crohn's Disease Patients | ClinicalTrials.gov. Access 11 April 2025.
- Neurath MF. Strategies for targeting cytokines in inflammatory bowel disease. Nat Rev Immunol. 2024;24(8):559-576. doi: 10.1038/s41577-024-01008-6.
- Atreya R, Abreu MT, Krueger JG, et al. Guselkumab, an IL-23p19 subunit-specific monoclonal antibody, bind CD64+ myeloid cells and potently neutralises IL-23 produced from the same cells. Presented ECCO 2023; P504.
- Rubin DT, Allegretti JR, Panés J, et al; QUASAR Study Group. Guselkumab in patients with moderately to severely active ulcerative colitis (QUASAR): phase 3 double-blind, randomised, placebo-controlled induction and maintenance studies. Lancet. 2025;405(10472):33-49. doi: 10.1016/S0140-6736(24)01927-5.
- Bressler B, Peyrin-Biroulet L, Afif W, et al. Guselkumab maintenance treatment improves health-related quality of life in patients with moderately to severely active ulcerative colitis: phase 3 QUASAR maintenance study. Presented at ECCO 2025; P1169.
- Peyrin-Biroulet L, Allegretti JR, Danese S, et al. Efficacy and safety of subcutaneous guselkumabinduction therapy in patients with Ulcerative Colitis: Results through week 12 from the phase 3 ASTRO study. Presented at ECCO 2025; OP10.
Disclaimer: This programme is not affiliated with ECCO.