ECCO News Editors’ Summary of the ECCO’19 Scientific Programme
Willem Bemelman, ECCO News Editor; Nuha Yassin & Ignacio Catalán-Serra, ECCO News Associate Editors
 Willem Bemelman Willem Bemelman © ECCO |
 Nuha Yassin Nuha Yassin © ECCO |
 Ignacio Catalán-Serra Ignacio Catalán-Serra© Ignacio Catalán-Serra |
ECCO’19 Copenhagen welcomed the record number of participants – 8034 – by offering the three-day Scientific Programme “Research drives clinical care”, 16 educational courses, and a poster exhibition of 860 hard copy posters, as well as a platform for 45 exhibitors and for innumerable business meetings.
One of these meetings also brought the new ECCO News Editors team together in order to plan for the publication year 2019.
 14th ECCO Congress at Copenhagen 2019 © ECCO 14th ECCO Congress at Copenhagen 2019 © ECCO |
 ECCO Booth at the 14th ECCO Congress © ECCO ECCO Booth at the 14th ECCO Congress © ECCO |
For this first issue of ECCO News, we aim to provide the ECCO News audience with a comprehensive summary of the ECCO’19 Scientific Plenary Programme by summarising all oral presentations and a selection of invited talks held in Copenhagen. This synopsis will be complemented by the upcoming publication of the traditional Talking Heads recording, which will be published on the eCCO-Learning Platform.
ECCO’19 also witnessed the launch of the brand-new ECCO e-Library, where you will shortly find all presentations and webcasts for which speakers granted publication consent together with the Congress abstracts, ECCO papers published in JCC, Y-ECCO Literature Reviews published in ECCO News and further tools.
The new e-Library facilitates user-friendly and high-quality search functions, building on a transparent indexing system for all search material as of ECCO’16.
Thematic categories used for the e-Library are aligned with the broad ECCO IBD Curriculum categories and constitute the backbone of the search function.
The predefined keywords ensure that the same system of terminology is used for roughly inventorying the whole content of the e-Library to facilitate free text search, which also applies to the abstracts.
 |
Summary of selected invited talks
Can we prevent IBD?
Jean-Fréderic Colombel, New York, United States
Jean-Fréderic opened the Scientific Programme with a mind-bending lecture on what he believed is the future of IBD management, namely the prevention of IBD. In his talk he focussed on secondary prevention, which means intervention in the healthy population that is identified as being at high risk of IBD. In the search for a screening tool, he used the DOD Serum Repository of more than 60 million serum samples of US Army soldiers. These samples were taken long before some of these soldiers developed IBD. Analysis and comparison with the healthy population showed that antimicrobial antibodies were elevated for a long period, even more than five years, before the disease became manifest. Also, different types of protein could be identified as associated with Crohn’s Disease. These proteins could even predict if the disease would present itself with a complication. Remarkably, this association was not found in UC. If the screening tool can be identified as a first step, then the target population can be selected. If positive, additional, more invasive tests can then be employed. The intervention finally could target the microbiome directly, or do so indirectly via a specific diet. Other interventions could target the innate immune system or the mucosal barrier. For sure, the intervention must be safe and reasonably effective. An example could be a Clostridium cocktail.
Management of IBD outside of Europe: The example of India
Rupa Banerjee, Hyderabad, India
Rupa took the audience on a trip outside of Europe. In an impressive talk, Rupa explained how the Western life-style came across India and brought IBD with it. There is an unmet need to identify the most appropriate management in India, taking into account disease presentation and cost in the country. Unresolved issues are the cause of the increasing incidence, diagnostic decision-making in relation to infectious disease, the management of optimisation of drug use, the cost issue and insufficient collaborative working. Rupa spoke about the problem of diagnosis when almost two-thirds of cases of bowel disease are infectious, with tuberculosis the number one mimic of Crohn’s Disease radiologically, endoscopically and histologically. Empirical use of tuberculostatics and antibiotics is therefore mandatory in managing the IBD patient. The consequences of the resultant diagnostic delay are more complications and greater need for surgery. There is also a huge cost issue, and most patients pay for the drugs out of their own pocket. Thiopurines have proved relatively cost effective, particularly at a low dose. A top-down strategy with biologicals is impossible. Accelerated step-up is the key. The exit strategy with respect to biologics is very important penny-wise. Limited resection for limited disease is an important strategy. Rupa discussed practical problems, including the fact that IBD is in general not considered a public health problem in India, the low level of physician awareness, the limited access to health care facilities and the issue of overlap with infectious diseases. The first results of the IBD emerging nations’ consortium from an inception cohort of 10,000 patients showed limited biological use, rare perianal disease and more extraintestinal IBD compared with the Western population. Rupa concluded that IBD in India is on the rise and that there is a (global) need for low-cost effective drugs. Overall it is a struggle to offer an adequate and effective healthcare service for all.
Can we cure IBD?
Arthur Kaser, Cambridge, United Kingdom
Arthur started his talk with a positive remark, that the landscape for management of disease can suddenly change if the cause of a disease is unravelled. Examples are the management of H. pylori, hepatitis C and melanoma. Then he elegantly said what everyone knows but does not dare to say out loud: our current therapeutics are not even close to the cause. Current medical strategies provide generic suppression of intestinal inflammation and relapse is almost inevitable after cessation of treatment: stopping treatment will result in recurrence. He argued that there is a great need for better understanding of the risk landscape. The epidemiology and relation between Westernization and IBD points to an environment–gene interaction as the cause. The environment is microbiota, which might be the true trigger, but the extreme diversity makes it very complicated. He said that there we are not even close to “the smoking gun” like H. pylori. He concluded by stating that we need this one critical discovery that will change everything.
Where is the exit?
Marc Ferrante, Leuven, Belgium
In his talk, Marc gave us some clear guidance on what to do in IBD management when thinking about the withdrawal of drugs. Withdrawal of immunomodulators is only advised if the disease has been stable for a long period, e.g. five years. Stopping the immunomodulator is associated with an annual disease relapse rate of 10%. He advised that when the immunomodulator is given as a part of combination therapy, the decision to stop the immunomodulator after 6–12 months should be based on the anti-TNF level. In the presence of a good anti-TNF level (>5), the immunomodulator could be stopped.
Withdrawal of anti-TNF is a different ball game. Here, relapse can be expected in half of patients after 2 years and probably in 85% with longer follow-up. The good news was that retreatment is associated with a 90% response rate, probably due to the maintenance of the immunomodulator in most of these cases. A risk factor for flare after anti-TNF withdrawal is clearly ongoing inflammation as demonstrated in stool or by endoscopy or imaging. Marc’s clear message was that ongoing inflammation must be ruled out before stopping the treatment, and that after cessation the patient must be followed up closely. He would not generally advise stopping a biological in the absence of specific reasons, such as the development of cancer or at the patient’s request.
Challenges in managing the elderly
Guillaume Savoye, Rouen, France
Guillaume warned the audience about the upcoming wave of elderly patients with IBD. He defined ‘elderly’ as being older than 60–65. He pointed out several challenges when treating the elderly, e.g. misdiagnosis with other types of colitis, the different natural history of the disease, the presence of comorbidities and the increased side effects of medical and surgical treatment. Particularly the very old (>80 years) are at increased risk of dying, and in this patient group acute colitis has a 25% three-month mortality risk. Other “killers” of the elderly with IBD are thrombo-embolic complications, secondary C. difficile infection and “us” as doctors prescribing polypharmacy. Therapy in the elderly is not different, but the risk profile of the drugs is. Systemic steroids should be avoided because of infectious complications, as should thiopurines in view of their cancer risk. Biologicals should be carefully used because of the risk of severe infections, particular pulmonary. There are, however, few data relating to the risk profiles of drugs in the elderly. There is certainly a clear increase in operative risk in the emergency setting. Timing of surgery is therefore essential.
Robot and surgeon: How will a partnership work?
Antonino Spinelli, Milan, Italy
Antonino Spinelli introduced the predominantly non-surgical audience to the future world of high-tech surgery. He predicted another change in the landscape of surgery. Surgery used to be handcraft with direct patient contact. Nowadays we are moving away from that by using tools that enable access to the body via small holes. If, in the old days, surgery involved fast execution with low precision, it is now slower but with high precision and less trauma. At the end of the last century, robotic systems were introduced. This master-slave device enables very precise manipulation. Spinelli predicted that the application of the robotic platform in surgery would rise from 1.8% to 10% in ten years’ time. The second-generation systems, however, will look different. They will comprise a digital integrated platform that helps the surgeon by integrating all the evaluable patient information. Spinellli predicted that future systems could learn from procedures through machine learning, making it possible to guide surgeons in their performance. Other interesting technologies were discussed, e.g. holographic technology to visualize anatomy during surgery and thereby help the surgeon. Other future tools were also considered: augmented vision using fluorescence techniques, automatic recognition and tagging of procedural steps, standardisation of procedures and easy data sharing. Thanks to 5G technology, real-time telemonitoring is now possible, enabling the instruction of surgeons at long range on how to do procedures.
 14th ECCO Congress at Copenhagen 2019 © ECCO 14th ECCO Congress at Copenhagen 2019 © ECCO |
 14th ECCO Congress at Copenhagen 2019 © ECCO 14th ECCO Congress at Copenhagen 2019 © ECCO |
Summary of Oral Presentations
OP01 In-depth characterisation of host genetics and gut microbiome unravels novel host–microbiome interactions in inflammatory bowel disease
Hu et al. presented new data regarding the interactions between host genetic factors and changes in the gut microbiota in IBD using whole-exome sequencing and metagenomics. The study showed how both common and rare genetic variants (like IL17REL, CYP2D6 or GPR151) affect the gut microbiome composition and function in the context of IBD. This work contributes to a better understanding of the interactions between the genome and the changes in the gut microbiome composition and function, contributing to intestinal inflammation.
Highlights of ECCO'19 recording - Part 1
OP02 The role of PTPN2 SNP in the pathogenesis of fibrosis in Crohn’s disease
Li et al. presented their work on the pathogenesis of fibrosis in Crohn’s Disease. They demonstrated a loss of function of PTPN2 gene variants associated with rs7234029 in primary cultures of human ileal subepithelial myofibroblasts of patients with stricturing Crohn’s Disease (Montreal B2). This alteration resulted in an excess of collagen I production and proliferation, contributing to the formation of strictures. This works highlights the importance of the genetic background in the pathogenesis of stricturing Crohn’s Disease.
Highlights of ECCO'19 recording - Part 1
OP03 Inhibition of autophagy exacerbates intestinal fibrosis and EMT
Cosin-Roger et al. studied the relevance of autophagy in intestinal fibrosis in Crohn’s Disease. The authors showed that the pharmacological inhibition of autophagy exacerbates murine intestinal inflammation, fibrosis and epithelial-to-mesenchymal transition (EMT) markers. In addition, the authors studied intestinal resections of Crohn’s Disease patients, showing that the expression of autophagy markers correlates with the expression of pro-fibrotic and pro-EMT genes. This work contributes to a better understanding of the importance of autophagy in the formation of intestinal strictures in CD.
OP04 Turning sweet in inflammatory bowel disease: glycans as novel immunomodulators of T-cell-mediated immune response
In this work, sponsored by an ECCO Grant in 2017, Dias et al. studied the role of glycans as immunomodulators in ulcerative colitis. They demonstrated that metabolic supplementation of ex vivo mucosal T cells extracted from active UC patients with glycan N-acetylglucosamine resulted in suppression of T-cell activity and growth and inhibition of Th1/Th17 response. The study also demonstrated that a deficiency in the branched N-glycosylation pathway exhibited increased susceptibility to severe forms of colitis in a mouse model. In addition, the levels of expression of branched N-glycans in colonic biopsies of UC patients predicted the failure to standard therapy. This study suggests that glycans could be used to modulate T cells response and inflammation.
Highlights of ECCO'19 recording - Part 1
OP05 Crohn’s disease exclusion diet is equally effective but better tolerated than exclusive enteral nutrition for induction of remission in mild-to-moderate active paediatric Crohn’s disease: a prospective randomised controlled trial
Van Limbergen et al. presented the results of a 12-week prospective, multicentre RCT exploring the anti-inflammatory effect of diet modifications for the treatment of paediatric Crohn’s Disease. The study compared the Crohn’s Disease exclusion diet (CDED) – a whole food diet coupled with partial enteral nutrition (PEN) – to exclusive enteral nutrition (EEN) in children with mild-to-moderate luminal CD. Both diets were effective and achieved high rates of corticosteroid-free sustained remission, with a significant decrease in inflammation measured by PCDAI and CRP. However, the CDED was better tolerated and showed higher sustained remission rates by week 12. These data support the use of CDED+PEN as a first-line therapy for children with luminal mild-to-moderate active CD and highlight the efficacy of diet modifications in paediatric CD.
Highlights of ECCO'19 recording - Part 2
OP06 Gut–brain axis revisited: Shedding light on the mucosa-associated microbial composition in IBD patients with psychological distress, anxiety, and depression
Humbel et al. aimed to elucidate a potential interplay between gut microbial composition and psychological status in a cohort of Swiss IBD patients. The authors found significant alterations in the intestinal mucosa-associated microbiome composition in relation to psychological well-being and quality of life. Alpha diversity was lower in patients with higher perceived stress and beta diversity was significantly different in IBD patients with vs. without depression or anxiety. Furthermore, specific relevant genera, like Proteobacteria, Firmicutes and Lactobacillales species, are significantly dysregulated in IBD patients with depression and anxiety. This study highlights the importance of the gut-brain axis in IBD.
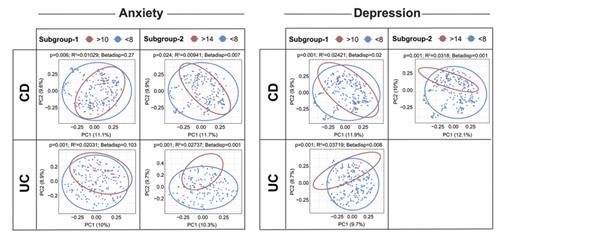 ECCO'19, OP06, Figure 1 |
OP07 Analysing intestinal organoids in a multi-omics, systems biology framework to investigate functional processes affected in Crohn’s disease due to autophagy impairment
Gul et al. investigated how autophagy impairment could affect key cell functions of Paneth cells contributing to the pathogenesis of CD, using mouse intestinal organoids. An integrated analysis combining proteomics and network biology showed that when autophagy is impaired, nearly 300 proteins display increased or decreased abundance, affecting at least 18 functional processes that affect Paneth cell functions. This work adds relevant information on the potential intestinal effects of autophagy-related mutations in CD patients.
OP08 Long-term efficacy and pharmacodynamics of the anti-mucosal addressin cell adhesion molecule-1 (MAdCAM-1) monoclonal antibody SHP647 in Crohn’s disease: the OPERA II study
D’ Haens et al. presented the results of the OPERA II extension study, which has aimed to assess the long-term safety and efficacy of SHP647 – a fully human IgG2 anti-mucosal addressin cell adhesion molecule (MAdCAM-1) antibody – in moderate-to-severe CD. This multicentre, open-label, Phase 2 extension study included patients who had completed the induction treatment in OPERA or in TOSCA studies. Dose reduction due to intolerance/adverse events or escalation due to clinical poor response was allowed from week eight. The remission rates were sustained over 72 weeks, regardless of initial response to induction treatment or dose escalation. hsCRP and faecal calprotectin levels were higher in patients who dose escalated. These results add important information on the long-term safety and efficacy of the MAdCAM-1 antibody SHP647 in patients with CD.
OP09 Histological remission and mucosal healing in a randomised, placebo-controlled, Phase 2 study of etrasimod in patients with moderately to severely active ulcerative colitis
Peyrin-Biroulet et al. presented the histological remission and mucosal healing results at week 12 in patients with moderately to severely active Ulcerative Colitis treated with etrasimod (APD334) from the OASIS Phase 2 study. Etrasimod is an oral, selective sphingosine-1-phosphate receptor modulator. Compared with placebo, etrasimod 2 mg resulted in significantly more patients achieving endoscopic improvement, mucosal healing and histological improvement/remission scored by a blinded central pathologist using the Geboes index. These results add important information on the efficacy of etrasimod in mucosal healing and histological improvement after induction at week 12 in UC.
Highlights of ECCO'19 recording - Part 3
OP10 Systems genomics of ulcerative colitis: combining GWAS and signalling networks for patient stratification and individualised drug targeting in ulcerative colitis
Brooks et al. aimed to study the pathogenic signalling pathways of Ulcerative Colitis to improve patient stratification. The authors used a novel systems biology workflow, iSNP, to identify regulatory disease-associated single-nucleotide polymorphisms (SNPs) and built a UC-interactome network. Taking advantage of this approach, the authors were able to analyse the regulatory effects of UC-associated SNPs both in a large cohort and at the individual level. Of interest, the authors identified MAML2, a Notch pathway activator, as a marker for therapeutic upscaling and disease severity in UC. This workflow can represent an interesting tool to use the genomic footprint to identify cohorts of patients who may benefit from specific therapeutic approaches.
OP11 Organoids derived from inflamed intestinal biopsies of patients with ulcerative colitis lose their inflammatory phenotype during ex vivo culture
To address whether ex vivo organoids obtained from Ulcerative Colitis patients retain the inflammatory character of their origin over time, Arnauts et al. compared patient-derived intestinal organoids created from both inflamed and non-inflamed regions of the colon of UC patients. The authors compared the transcriptome of whole biopsies, crypts and ex vivo cultured organoids after one and four weeks. This comparison showed that organoids derived from inflamed crypts lost part of their inflammatory character after one week in culture. Furthermore, organoids derived from inflamed and non-inflamed biopsies were no longer distinguishable after four weeks in culture. The authors concluded that it is not essential to obtain biopsies from inflamed regions to culture organoids from UC patients and suggested the importance of adding the immune cells to the ex vivo culture system to mimic a physiological representative model of intestinal inflammation.
OP12 Targeting inflammation in ulcerative colitis by inhibiting glucose uptake
During inflammation, the inflammatory cells switch their metabolism from lipid oxidation to glycolysis to ensure quick activation, proliferation and migration. In this work, Gropp et al. tested ability of the PI3K inhibitor copanlisib and the glucose uptake inhibitor ritonavir to control inflammation, both in vitro and in vivo (NSG-UC mouse model). The authors showed an effect on activated CD4+ with both copanlisib and ritonavir. This work suggests the modulation of T cell metabolisms to control inflammation. The authors proposed that approved drugs like copanlisib and ritonavir could be tested against human intestinal inflammation.
OP13 Molecular response to ustekinumab in moderate-to-severe ulcerative colitis by serum protein and biopsy gene expression analysis: Results from the UNIFI Phase 3 induction study
Li et al. presented results on the molecular effects of ustekinumab in UC patients. Colonic biopsy mRNA and serum samples from patients included in the UNIFI Phase 3 induction study were studied. Normalisation of IFN-γ, SAA, IL-17A and IL-22 was first detected in responders to ustekinumab at week 4 and continued to improve through week 8. However, TNF was elevated prior to treatment and was not normalised by ustekinumab induction therapy. This wide study using transcriptomic and protein analyses demonstrated the suppression of IL-12 (IFN-γ) and Il-23 (IL-17A) pathways and normalisation of the UC disease gene expression profile in response to ustekinumab.
OP14 Improved endoscopic outcomes and mucosal healing of upadacitinib as an induction therapy in adults with moderately to severely active ulcerative colitis: data from the U-ACHIEVE study
Sandborn et al. presented the results of the analysis of the endoscopic and histological effects of upadacitinib in Ulcerative Colitis patients included in the U-ACHIEVE study. U-ACHIEVE is a Phase 2 study designed to assess the efficacy and safety of upadacitinib – an oral, selective Janus kinase 1 inhibitor – in patients with moderately to severely active UC. The proportion of patients achieving endoscopic improvement, endoscopic remission, histological improvement, histological remission and mucosal healing at week 8 was statistically significantly higher in the upadacitinib 30 and 45 mg QD groups vs. the placebo group. This work supports the efficacy of a high dose of orally administered upadacitinib to induce endoscopic and histological improvement in moderate to severe UC.
OP15 Cost analysis in a prospective European population-based inception cohort: is there a cost-saving effect of biological therapy?
Burisch et al. presented the results of a prospective long-term analysis of healthcare costs in patients with IBD in the era of biological treatments. The aim of this study was to perform a cost analysis of a pan-European inception cohort (Epi-IBD) including 20 European countries, with five years of follow-up. The authors showed an increased expenditure on biological therapy in this time period in both Western and Eastern Europe. However, this was paralleled by a steady decrease in the costs of non-biological standard treatments, hospitalisation and surgery. The authors conclude that these results indicate a cost-saving effect of biological medications.
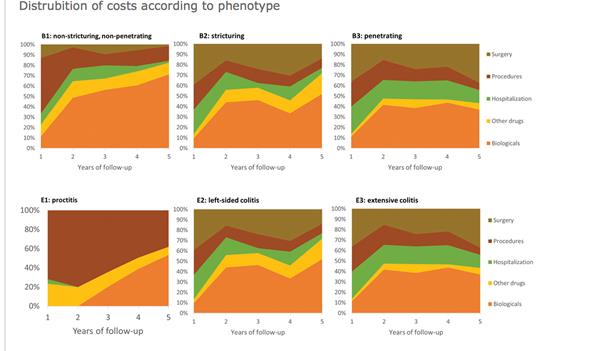 ECCO'19, OP15, Figure 2 |
OP16 A randomised, multi-centre, double-blind, placebo-controlled study of a targeted release oral cyclosporine formulation in the treatment of mild-to-moderate ulcerative colitis: efficacy results
Bloom et al. presented the results of a pilot trial exploring the efficacy of a targeted release oral cyclosporine formulation (ST-0529) in the treatment of mild-to-moderate Ulcerative Colitis. 118 subjects were included in this four-week induction Phase 2a randomised, multicentre, double-blind, placebo-controlled study. ST-0529 was safe and well tolerated and a numerical (although not statistically significant) advantage of ST-0529 over placebo was found for rates of clinical remission and clinical response. However, no differences were found between the treatment groups with regard to mucosal and histological healing. In a post-hoc analysis, the authors reported the largest clinical response rate in moderate UC patients taking 5-aminosalicylates and/or steroids.
OP17 A molecular measure of inflammation in IBD patients based on transcriptional profiles from 2495 intestinal biopsies
In this study, Huang et al. explored the use of whole transcriptome gene expression to define molecular scores of gut inflammation. A molecular characterisation of IBD based on the transcription profiles of a large number of inflamed and non-inflamed intestinal biopsies was performed and a molecular inflammation score (MIS) via gene set variation analysis was generated. MIS scores were strongly associated with features captured by histological, endoscopic and clinical activity, adding a broader range of inflammation signal than histological assessment. The authors conclude that MIS may improve patient classification, identify subclinical disease and predict flares or therapeutic response. This work shows how the molecular characterisation of both UC and CD patients could be useful as a biomarker, adding relevant information to the currently used outcome predictors.
OP18 Proactive adalimumab trough measurements increase corticosteroid-free clinical remission in paediatric patients with Crohn’s disease: The paediatric Crohn's disease adalimumab level- based optimisation treatment (PAILOT) trial
A. Assa and colleagues presented the results of this multicentre, non-blinded, randomised, controlled trial, in which children (6–18 years) with luminal Crohn’s Disease who were naïve to biological therapy and responded to adalimumab induction (week 4) were randomly assigned into proactive and reactive follow-up groups. Eighty patients were divided into two groups. Trough concentrations were measured at weeks 4 and 8 and then every eight weeks until week 72 in the proactive group. In the reactive group, the trough levels were measured only when clinically indicated (based on symptoms or elevated CRP or faecal calprotectin) and dose/intervals were adjusted based upon these levels. The primary endpoint was sustained corticosteroid-free clinical remission from week 8 to week 72 [defined as a Paediatric Crohn’s Disease Activity Index (PCDAI) score <10] using non-responder imputation. This primary endpoint was met by 34 children (87%) in the proactive group and 21 (49%) in the reactive group (p<0.001. Given the ability to achieve higher corticosteroid-free sustained remission rates, the study supports the use of repeated proactive trough measurements together with tight control based on clinical indices, CRP and faecal calprotectin.
OP19 Corticosteroid response rectal gene signature and associated microbial variation in treatment naïve ulcerative colitis
The PROTECT study investigated newly diagnosed paediatric patients with UC. The results from the PROTECT study showed that the strongest predictor of corticosteroid (CS)-free remission by week 12 or 52 was week 4 (WK4) remission. In this sub-study, the group aimed to define the key pathways linked to WK4 response to standardised induction with CS in the largest prospective paediatric UC cohort to date. Pre-treatment rectal biopsies were used. mRNA-Seq and 16S rRNA defined pre-treatment rectal gene expression and microbial communities in 206 participants. An independent group of 50 participants was used to validate the CS response gene signature. WK4 remission was defined as PUCAI <10 without additional therapy/colectomy. 115 genes were differentially expressed (FDR<0.05 and FC ≥1.5) between moderate–severe UC patients who did or did not achieve WK4 remission in the discovery cohort. The corticosteroid response gene signature was highly associated with CXCR chemokines (p<7.12E−12), innate myeloid immune signatures (p<1.62E−15), and response to bacteria (p<2.16E−13). The addition of the pre-treatment rectal gene signature PC1 [OR=0.4 (95% CI 0.2–0.8)] improved the WK4 clinical prediction model of remission with CS [AUC=77.7 (95% CI 70.0–85.4)].
The group identified a gene signature linked to WK4 CS response, which was validated in independent UC patients, and showed associations with response to anti-TNFα and anti-α4β7 integrin in adults, and with specific microbial taxa. These data may assist in the prioritisation of future therapies for non-responders over current approaches.
OP20 Mucosal microRNA profiles predict response to autologous stem-cell transplantation in Crohn’s disease
Following the recent Autologous Stem Cell Transplantation for Crohn’s Disease (ASTIC) trial, the work from this multicentre group from the UK aimed to explore the ability of microRNAs to predict response to haematopoietic stem cell transplantation (HSCT) in patients with CD. miRNA profiles were analysed in RNA extracted from mucosal biopsies taken prior to HSCT from 14 CD patients enrolled in ASTIC. Clinical response to therapy was defined as CDAI <150 at 1 year; the cohort included seven ‘responders’ and seven ‘non-responders’. Two groups were identified via PCA: group 1 contained six of the seven responders and group 2 contained six of the seven non-responders. Significant separation of responders and non-responders was identified along principal component 2. Inspection of the loadings for PC 2 identified miR-155-5p as a significant contributor to the separation of the groups. Levels of miR-155-5p were significantly higher in non-responders relative to responders (p=0.033). The area under the ROC curve for miR-155-5p was 0.877, indicating that response to therapy could be accurately predicted in 87.7% of patients from their basal miR-155-5p levels.
This study indicates that miRNAs may act as predictive biomarkers of clinical response following HSCT. In particular, miR-155-5p, a well-characterised pro-inflammatory miRNA, was identified as a putative candidate biomarker. We await independent validation of these exciting results.
OP21 ABX464 is safe and efficacious in a proof of concept study in Ulcerative Colitis patients
This was a Phase 2a study performed in 15 European centres in Belgium, France and Poland. It is a first-in-disease study using ABX464 in patients with moderate-to-severe Ulcerative Colitis intolerant and/or refractory to existing treatments. ABX464 has potent anti-inflammatory properties that impact the expression of miR124, as shown in HIV studies. A total of 32 patients were randomised (2:1) to ABX464 50 mg QD orally or placebo for eight weeks. The primary endpoint was safety of ABX464 and key secondary endpoints included remission (assessed as a rectal bleeding sub-score = 0 and an endoscopy sub-score ≤1 and at least a one-point decrease in stool frequency sub-score from baseline to achieve a stool frequency sub-score ≤1), endoscopic improvement (Mayo endoscopic score of 0 or 1), and clinical response and histological healing. Centrally read endoscopy with histopathology was performed at day 0 and day 56. After the blinded induction phase, patients had the option to roll over into a 52-week open-label 50 mg QD ABX464 study.
A total of 29 (90.6%) patients (20 randomised to ABX464 and 9 to placebo) completed the induction study. The overall safety profile of ABX464 was very good, with no serious adverse events. Twenty-two patients were included in the 52-week maintenance study. The interim analysis with a mean maintenance treatment duration of 5.1 months (max: 9.0 months; min: 3.5 months) showed further improvement in both the partial Mayo score and faecal calprotectin levels.
In patients with moderate-to-severe UC, ABX464 50 mg QD orally for eight weeks was shown to be safe and well tolerated. Clinical, endoscopy, histopathology and biomarker analysis all changed favourably after its use. We eagerly await further studies using this drug.
OP22 Mesenchymal stromal cell-derived exosomes stimulate epithelial regeneration in vitro and reduce experimental colitis
Mesenchymal stromal cells (MSCs) are generally believed to work by modulation of immune responses and stimulation of tissue regeneration. They are thought to communicate with neighbour cells through secreted proteins and via direct cell-to-cell contact. Recent literature shows that they can also communicate via MSC-derived exosomes. This group from the Netherlands investigated the effect of MSC–exosomes on epithelial regeneration and whether local MSC therapy in experimental colitis could be mediated by MSC-derived exosomes. They also explored MSC–exosome therapy as a cell-free alternative for MSC therapy. Exosomes were isolated from bone marrow-derived murine MSCs using ultracentrifugation. The presence of exosomes was verified using electron microscopy and western blotting for the exosome markers flotillin-1 and alix. The group showed that a high dose of MSC-derived exosomes is able to counteract epithelial damage in vitro and partially reduce colitis in vivo. This study provides very useful data to support further studies exploring cell-free MSC-related therapy by using MSC–exosomes in the treatment of IBD.
OP23 CKD-506, a novel histone deacetylase (HDAC) 6 inhibitor, ameliorates colitis in various animal models
Inhibition of HDAC6 has been proposed to be beneficial and to have therapeutic effects in Inflammatory Bowel Disease. This study focussed on identifying the molecular mechanisms underlying the therapeutic effect of CKD-506, an oral selective HDAC6 inhibitor, in various colitis human and animal models.
HDAC6 expression was assessed in colon tissue of healthy individual and patients with Crohn’s Disease and Ulcerative Colitis by real-time RT-PCR and immunohistochemistry. Macrophages with HDAC6 overexpression were used for mechanism studies. DSS-, TNBS-, piroxicam (IL-10−/−)- and adaptive T-cell transfer (RAG1−/−)-mediated colitis animal models were also used to check the efficacy of CKD-506. Colitis animals were treated with 1–100 mg/kg of CKD-506 and various disease activity indexes were analysed, such as body weight, colon length, cytokines in serum, colon tissue and lamina propria mononuclear cells (LPMC).
HDAC6 was overexpressed in colon tissue of patients with Crohn’s Disease and Ulcerative Colitis. In vitro, HDAC6 overexpression by pDNA strongly induced the production of various inflammatory mediators, especially TNFα, IL-6, IP-10 and ROS production from macrophages. However, CKD-506 inhibited HDAC6-mediated inflammatory responses in macrophages through NF-kB and AP-1.
In vivo, CKD-506 strongly inhibited disease activity indexes in DSS-, TNBS-, piroxicam- (IL-10−/−)- and adaptive T-cell transfer-mediated colitis. In acute colitis models, CKD-506 inhibited IL-6 and TNFα expression in colon tissue of DSS-induced colitis and also inhibited ICAM-1, VCAM-1 and IP-10 expression in colon tissue of the TNBS-induced colitis model. In addition, CKD-506 inhibited Ik-B phosphorylation, IL-6 and TNFα expression in colon tissue and mononuclear cells of lamina propria in piroxicam-induced Colitis of IL-10−/− mice. CKD-506 inhibited various inflammatory cytokines in serum as well as in colon tissue of T-cell adaptive transfer colitis of RAG−/− mice.
The data have shown that inhibition of HDAC6 by CKD-506 has a therapeutic effect in colitis animal models. The use of CKD-506 may have some beneficial effects in patients with Crohn’s Disease and Ulcerative Colitis.
OP24 Effectiveness and safety of ustekinumab 90 mg every 4 weeks in Crohn’s disease
The most commonly used drug regimen for ustekinumab in Crohn’s Disease (CD) is 90 mg every 8 weeks. Some patients will partially respond to ustekinumab or will experience a secondary loss of response. These patients might benefit from shortening of the interval between injections. This collaborative French group aimed to study the efficacy and safety of ustekinumab 90 mg every four weeks (90 mg q4W). The study was a retrospective multicentre cohort study which included all patients with active CD, as defined by CDAI >150 and one objective sign of inflammation (CRP >5 mg/l and/or faecal calprotectin >250 µg/g and/or radiological and/or endoscopic evidence of disease activity) who required ustekinumab dose escalation to 90 mg q4W for loss of response or inadequate response to ustekinumab 90 mg q8W. This is the first study of its kind.
Seventy-six patients were included. Optimisation was performed after a median of 4.5 months (IQR, 2.2–7.2) of ustekinumab treatment initiation.
Two-thirds of patients recaptured response following treatment optimisation. Colonic location, inflammatory behaviour and duration of ustekinumab therapy before optimisation were associated with clinical response at two months. At the end of follow-up, 35% (n =27/76) had been hospitalised, and 22% (n=17/76) had undergone surgery. Adverse events were reported in 9% (n=7/76) of patients, including two serious adverse events (pneumonitis, infectious colitis). This study provides valuable data on the use of ustekinumab in CD.
OP25 Targeting endoscopic outcomes through combined pharmacokinetic and pharmacodynamic monitoring of infliximab therapy in patients with Crohn’s disease
This is a follow-on study from the TAILORIX study looking at the monitoring of infliximab (IFX) in CD. The initial results of TAILORIX suggested that trough concentrations (TC) >23.1 mg/l at week 2 and >10.0 mg/l at week 6 predicted endoscopic remission (CD Endoscopic Index of Severity <3) at week 12. During maintenance therapy, no exposure–response relation was observed, but faecal calprotectin (FC) was lower in patients achieving the endoscopic outcomes compared with patients who did not.
The estimated IFX clearance (CL) during induction therapy was lower in patients achieving endoscopic remission at week 12, which was not observed during maintenance therapy. During maintenance therapy, an exposure–response relationship was observed only after dose escalation, with a TC >10.8 mg/l after dose escalation predicting the absence of ulcers at week 54. This exposure–response relation only appeared after three infusions at the elevated dose. In patients with elevated FC (>250 mg/kg), a significant drop was observed immediately upon dose escalation, resulting in FC concentrations that were lower in patients without ulcers compared with patients with ulcers. Antibodies to IFX (ATI), measured using a drug-tolerant assay, increased IFX CL by ~48%, resulting in a reduction of the terminal half-life from 9.4 to 6.4 days.
The study group recommend proactive and reactive monitoring of FC during IFX maintenance therapy. If/when FC does not normalise upon dose escalation, it was suggested that the IFX TC would provide information on the mechanism of failure and could thus guide clinical decision-making.
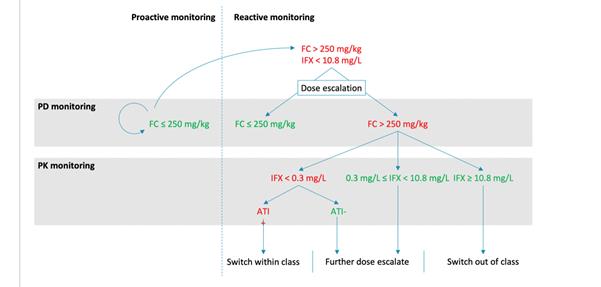 ECCO'19, OP25, Figure 2 |
OP26 Long-term safety of vedolizumab in ulcerative colitis and Crohn’s disease: final results from the GEMINI LTS study
GEMINI long-term safety (LTS) was the longest vedolizumab (VDZ) continuous treatment, multinational, multicentre, open-label Phase 3 study to date. The study group reported the final GEMINI LTS safety and efficacy findings.
A total of 894 patients with UC and 1349 with CD enrolled in GEMINI LTS for a planned treatment duration of nine years. Patients received VDZ 300 mg IV every 4 weeks after they completed or withdrew from a Phase 2 study or one of the GEMINI Phase 3 studies or enrolled as VDZ-naïve de novo patients. Treatment continued until study completion or loss to follow-up (e.g. after VDZ approval or expanded-access programme availability at the local site). Long-term safety was the primary endpoint and efficacy was an exploratory endpoint.
All patients had received ≥1 prior conventional therapy. Adverse events (AEs) occurred in 93% of UC patients and 96% of CD patients, the most frequent being UC (36%) and CD (35%) exacerbations and nasopharyngitis (UC, 28%; CD, 25%). Serious AEs (SAEs) were reported in 31% of UC patients and 41% of CD patients; disease exacerbation was the most frequent SAE in both cohorts (UC, 13%; CD, 17%). VDZ was discontinued due to AEs in 15% of UC patients and 17% of CD patients, with UC or CD exacerbation (9% and 8%, respectively) the most frequent reason for discontinuation. There were no cases of progressive multifocal leukoencephalopathy and ten (UC, 4; CD, 6) deaths during the study. Clinical response was maintained long-term in patients who continued to receive VDZ throughout the entire study; however, the efficacy analysis was limited due to the expected, protocol-defined patient loss to follow-up.
The final GEMINI LTS results provide evidence that VDZ has a safety profile suitable for long-term treatment of UC and CD, with no new trends observed for infections, malignancies, infusion-related reactions or hepatic events.
OP27 High-dimensional mass cytometry reveals the immune cell landscape in inflammatory bowel disease
This study group sought to explore a more comprehensive analysis of the cell composition in intestinal biopsies from IBD patients across all major immune lineages simultaneously, as data are lacking. Paired biopsies were taken from 104 patients, aged 10–40 years, from the ileum and colon (both inflamed and uninflamed mucosa) and blood samples from 23 IBD patients and 15 controls with a normal colonoscopy. Single-cell suspensions were stained with a 36-antibody panel and analysed with mass cytometry. A total of 309 distinct cell clusters were identified from the collective intestinal dataset containing 3.4 million cells in a data-driven manner. Affected samples from the different subgroups of IBD (Crohn’s Disease, Ulcerative Colitis, Indeterminate Colitis) were mostly intermixed, suggesting similarities in the immune profiles. Moreover, we observed a large interindividual variation in the immune cell composition, indicative of unique individual ‘immune fingerprints’ in the intestinal tract. Nineteen subsets were significantly different between affected-IBD samples and unaffected-IBD samples/controls. Additionally, in a correlation analysis, several CD4+ T-cell clusters correlated with ILC and myeloid cell clusters and were up-regulated in IBD-affected segments, while in particular TCRgd cell clusters and a group of ILC clusters were up-regulated in unaffected samples of patients and controls. The study provided evidence that a coordinated cellular network of both innate and adaptive immune cell types is implicated in IBD. Together with the evidence for the unique individual-specific composition of the intestinal immune system, this may aid in the development of more (cost-effective) and personalised patient care in the future.
OP28 Host–microbial crosstalk in the pathogenesis of inflammation and cancer in primary sclerosing cholangitis
This group aimed to assess the host–microbial functions in PSC-IBD and evaluate whether PSC-IBD-associated pathways affect epithelial transformation. Biopsies and mucosal brushings were taken from the colon and terminal ileum of patients with PSC-IBD, Ulcerative Colitis without PSC (UC) and healthy controls (HC). 3′RNA sequencing was performed to analyse intestinal transcriptomes and 16S rRNA sequencing to characterise the adherent microbiome. Colonic crypts were isolated from biopsies, seeded onto basement membrane extract and cultured in media containing growth factors to develop organoids. Organoids were stimulated with different cytokines for 24 h and markers of cytokine downstream pathways, stemness and pluripotency were analysed by qPCR. A distinct transcriptomic profile in the caecal biopsies of patients with PSC-IBD compared with UC and HC was identified, with 890 genes being regulated in PSC-IBD The transcriptomic profile in the colonic mucosa of patients with PSC-IBD showed altered regulation of pathways previously associated with IL22 and TGFβ signalling. Both cytokines have been implicated in cancer pathogenesis. PSC-IBD-associated Th1 responses may result in increased epithelial IL22 responsiveness. Higher expression of the cancer stemness genes OLFM4 and POU5F1, triggered by bacteria and IL22 via STAT3 activation, suggests that microbial-driven IL22 responses may contribute to epithelial transformation.
OP29 ST2+/IL-33 responsive cells promote tumorigenesis in colitis-associated colorectal cancer
The aim of this study was to characterise the precise contribution of IL-33/ST2 axis in the azoxymethane (AOM)/dextran sodium sulphate (DSS) model of colitis-associated CRC. C57/BL6 wild-type (WT), IL-33 KO, ST2 KO and CD73 KO mice were given a single dose of AOM (7.4 mg/kg) followed by two cycles of 3% DSS for 7 days in drinking water. A disease activity index (DAI) was used and endoscopic and histological evaluations of colons were performed.
Immunohistochemistry, immunofluorescence and qPCR were done on full-thickness colons for IL-33 and ST2 localisation and identification and for analysis of mRNA expression. FACS analysis was performed on cell suspensions from resected, isolated polyps, and qPCR for vimentin, desmin, αSMA, CD34, CD31 and CD73 was completed on sorted cells in order to functionally characterise ST2+/IL-33 responsive cells.
The group identified that IL-33, ST2L and sST2 mRNA transcripts were dramatically elevated in AOM/DSS-treated WT mice vs. controls. The results suggested that the IL-33/ST2 axis promotes tumorigenesis in colitis-associated CRC through the activation of CD73.
OP30 Serum proteomic profiling predicts and diagnoses pouchitis in ulcerative colitis patients undergoing ileal pouch-anal anastomosis
This is an interesting study investigating pouchitis, which is the most common complication in patients with Ulcerative Colitis (UC) requiring ileal pouch anal anastomosis (IPAA). Pouchoscopy remains the gold standard to diagnose pouchitis in the absence of other surrogate biomarkers. The study group performed serum proteomic profiling to identify biomarkers that could be predictive and discriminative for development of pouchitis following IPAA.
A prospective cohort study of 51 patients undergoing IPAA was conducted at a single centre (46 UC and 5 familial adenomatous polyposis patients). Serum was collected before colectomy and at predefined clinical visits at months 1, 3, 6 and 12 after IPAA. At every clinical visit, patients had endoscopic evaluation of the pouch. Pouchitis was defined by the presence of endoscopic inflammation. Serum samples from 62 age- and sex-matched healthy subjects (HS) served as controls. A panel of 91 inflammation-related proteins was measured using the Proximity Extension Assay.
A total of 17 (37%) UC patients were diagnosed with pouchitis during the first year after IPAA. Younger age at colectomy and backwash ileitis were associated with pouchitis. The combination of HGF, TNFRSF9 and age at colectomy was the most accurate in predicting development of pouchitis within 1 year (AUC=0.875). A panel of four proteins (IL17A, CXCL1, CCL25 and TRAIL) showed a good discriminative power (AUC=0.984) to diagnose pouchitis at month 12 post IPAA.
The study concluded that before colectomy, there is a great overlap in serum protein profiles between patients who do and patients who do not develop pouchitis. Proteins involved in NK cell chemotaxis and cellular extravasation were dysregulated solely in patients developing pouchitis. HGF and TNFRSF9 in combination with age at colectomy were predictive for pouchitis, and we identified a combination of four biomarkers with diagnostic potential. This study has great potential in the future, and further validation in a larger cohort is required.
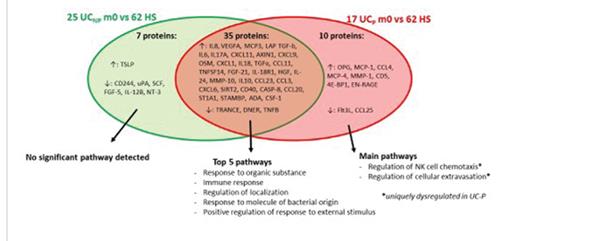 ECCO'19, OP30, Figure 1 |
OP31 TP53 mutation in human colonic organoids acquires resistance to in vitro long-term inflammation
In colitis-associated cancer (CAC), tumour protein p53 (TP53) mutation often occurs in the early phase of colon carcinogenesis known as the dysplasia–carcinoma sequence. Although there are some reports about the relation between TP53 mutation and colon carcinogenesis in mice models, the function of TP53 mutation on colonic epithelial cells in patients with Inflammatory Bowel Disease (IBD) has remained unknown.
The study group aimed to assess the influence of TP53 mutation by using a CRISPR Cas9 system on human colon epithelial organoids under a long-term inflammation model which the group had previously generated. TP53 mutation was established in three different human colon epithelial organoids. Mutant TP53 was strongly expressed in nuclei, as often shown in dysplastic lesions of IBD, whereas wild type (WT)–TP53 was not expressed in naive organoids. The effect of mutant TP53 was assessed with or without inflammatory stimulation for 60 weeks. Long-term inflammation impaired cell proliferation and sphere formation of the organoids with WT-TP53. Mutant TP53, however, enhanced cell growth and stemness with increased gene expression of c-myc and Lgr5 compared with WT–TP53 under the inflammatory situation; nevertheless, inflammatory response in the organoids with mutant TP53 was equal to that in the organoids with WT-TP53.
This study has shown that TP53 mutation maintains cell proliferation and stemness of human colonic organoids even under in vitro long-term inflammation. Mutant TP53 acquired resistance to cell damage by chronic inflammation, suggesting that these results might mimic cell phenotype at the early step of colitis-associated carcinogenesis. The take-home message is perhaps a new therapeutic target.
OP32 A novel mechanism of colonic epithelial-T-cell cross-talk is dysregulated in IBD
Epithelial dysfunction is an early initiating factor of Inflammatory Bowel Disease (IBD), yet the immunological consequences of this remain enigmatic. Juxtaposed to epithelial cells are specialised intraepithelial γδ T cells, implicated in maintaining tissue integrity. We have shown that specific members of the butyrophilin-like (BTNL) protein family are restricted to intestine epithelial cells and are profound γδ T-cell regulators. Thus, Btnl1−/− mice show selective depletion of a signature intestinal γδ subset. This axis is conserved in humans, where signature colon-resident, Vγ4+ γδ T cells are selectively regulated by direct interactions between their TCRs and a BTNL3/8 heteromer.
The study group investigated whether such selective regulation of human colonic γδ cells by BTNL3 + 8 is perturbed in IBD and examined factors which may modulate this. They described a novel and important axis by which epithelial cells maintain homeostasis of the γδ T cell compartment, and which is frequently dysregulated in IBD. The findings of this study may support the use of IL-12 blockade in restoring this axis whilst IL-23 may be redundant in this setting, with implications for future therapeutic strategies. Therapeutic blockade of αΕβ7 has the potential to disrupt an important axis in the human colon, which may exacerbate disease given the precociously active, pro-inflammatory nature of αΕβ7− γδ T cells. This is an interesting finding considering the therapeutic targets used in medical therapies at the moment.
OP33 BUB1: a new player in the development of Crohn's disease (CD)-associated fibrosis
The lack of efficient and well-tolerated anti-fibrotic drugs targeting intestinal fibrosis in CD is partly due to the fact that the main and specific cellular and molecular pathways leading to fibrosis remain to be identified. This study, from Humanitas in Milan, aimed to investigate whether BUB1 (budding uninhibited by benzimidazoles-1) is involved in the pathogenesis of CD-associated fibrosis. BUB1 is a serine/threonine-protein kinase that is essential for spindle-assembly checkpoint signalling, but it has recently been found to mediate TGFβ-dependent signalling. The group took surgical specimens from normal individuals, fibrostenotic CD patients and fibrotic patients with no signs of inflammation. The RNA was reverse transcribed and deep-sequenced. BUB1 expression and localisation were confirmed by quantitative real-time PCR. BUB1 expression levels were increased within the muscularis mucosa and muscularis propria of inflamed tissues in comparison with non-inflamed specimens, particularly when fibrosis was evident. BUB1 inhibition remarkably reduced collagen deposition in colitic mice and significantly improved TNBS-induced chronic colitis both clinically and histologically. In vitro, BUB1 inhibition reduced collagen deposition and the growth rate of CD-isolated myofibroblasts in comparison with vehicle-treated cells. This is, yet again, a study with very exciting results indicative of a potential therapeutic target for intestinal fibrosis associated with CD.
OP34 VARSITY: A double-blind, double-dummy, randomised, controlled trial of vedolizumab versus adalimumab in patients with active ulcerative colitis
>VARSITY is the first randomised controlled trial of its kind to directly compare two biological agents in the treatment of IBD. A European and American multicentre phase 3b, double-blind, double-dummy, randomised clinical study set out to compare the efficacy and safety of vedolizumab (VDZ) and adalimumab (ADA) for treatment over 52 weeks in adults with moderately to severely active Ulcerative Colitis (UC).
Patients with moderately to severely active UC (Mayo score 6 to 12 and endoscopic sub-score ≥2) who had failed other conventional therapies were included. Prior tumour necrosis factor (TNF) antagonist exposure was capped at 25% of the patient population. Patients were randomised 1:1 to either: (1) active VDZ intravenous (IV) infusions (300 mg)/placebo subcutaneous (SC) injections; or (2) placebo IV infusions/active ADA SC injections (160/80/40 mg). Dose escalation was not permitted in either group. The primary endpoint was clinical remission, defined as a complete Mayo score ≤2 with no sub-score >1 at week 52. A total of 769 patients were randomised to VDZ (n=383) or ADA (n=386) at 330 sites in 37 countries and received at least one dose of study drug. VDZ showed significantly better rates of clinical remission (primary endpoint) and mucosal healing. Overall clinical remission rates at week 52 were 31.3% (n=120/383) for VDZ and 22.5% (n=87/386) for ADA.
VDZ was superior to ADA in achieving clinical remission and endoscopic mucosal healing at week 52, while VDZ and ADA were both generally safe and well tolerated in patients with moderately to severely active UC.
OP35 Endoscopic and deep remission at 1 year prevents disease progression in early Crohn’s disease: long-term data from CALM
This study aimed to describe the long-term impact of achieving endoscopic and deep remission among participants in the effect of tight control management on CD (CALM) trial. The study group analysed the medical records of patients with follow-up data since the end of CALM. Patients were stratified by outcomes in CALM at 1 year: clinical remission (Crohn’s Disease Activity Index, CDAI <150), endoscopic remission (Crohn’s Disease Endoscopic Index of Severity, CDEIS <4 with no deep ulcerations) and deep remission (CDAI <150, CDEIS <4 with no deep ulcerations, and no steroids for ≥8 weeks). The primary outcome was a composite of major adverse outcomes reflecting CD progression: new internal fistula/abscess, stricture, perianal fistula/abscess, CD hospitalisation or CD surgery since end of CALM. One hundred and twenty-two patients were included. The study identified early CD patients who achieve endoscopic or deep remission after one year of intensive treatment to be less likely to have disease progression over a median of three years. These are encouraging follow-up data.
OP36 A colonic gene expression signature predicts non-response to anti-inflammatory therapies in inflammatory bowel disease
This study group had previously described PROgECT, a Phase 2a open-label study of patients with moderate-to-severe Ulcerative Colitis (UC), which prospectively validated the ability of a molecular profile score (MPS) consisting of a colonic 13-gene expression panel to predict response to TNF-antagonist therapy. For this study, their aim was to evaluate whether the MPS could be a useful tool in accurately identifying a subset of non-responders to therapy. The sensitivity and specificity of the MPS in identifying non-responders to therapy were evaluated in four independent TNF-antagonist trials (ACT1, PURSUIT-SC, PROgECT, PURSUIT-J) and an anti-IL12/23 trial (UNITI). The group also characterised the gene expression and microbiome profiles of those patients predicted to be non-responders by the MPS using microarray and 16S sequencing in the PROgECT cohort.
The findings showed the MPS to consistently predict non-responders to therapy in IBD regardless of ethnicity or whether the therapy targeted TNF or IL12/23 pathways. In addition to clinical parameters and inflammatory markers, MPS serves as the first prospectively validated predictive biomarker that can accurately identify a discrete subset of non-responders to two different anti-inflammatory therapies and may be valuable in identifying subsets of patients in need of treatment with alternative therapies or for patient stratification in clinical trials.
OP37 Efficacy and safety of ustekinumab as maintenance therapy in ulcerative colitis: Week 44 results from UNIFI
The study group planned to evaluate the safety and efficacy of SC ustekinumab (UST) as maintenance therapy in UC patients who were in clinical response to a single IV induction dose of UST. They designed a phase 3, double-blind, randomised withdrawal study in patients with moderate–severe active UC who failed conventional or biologic therapy (including anti-TNF and/or vedolizumab) and were in clinical response eight weeks after receiving a single UST IV induction dose.
The primary study population included 523 patients randomised 1:1:1 to placebo (PBO) SC, UST 90 mg SC q8w or q12w at week 0. Primary endpoint was clinical remission at week 44 (52 weeks after IV induction); key secondary endpoints were maintenance of clinical response, endoscopic healing, corticosteroid-free clinical remission and maintenance of clinical remission among patients who achieved clinical remission at baseline.
Both UST 90 mg q8w and q12w SC achieved clinical remission and maintained clinical response and were effective in achieving endoscopic healing and corticosteroid-free remission amongst patients with moderate–severe UC induced into clinical response with a single IV dose of UST. The safety for UST in UC patients was consistent with the known safety profile of UST in CD. These results are encouraging in the current management of UC and CD.
OP38 Maintenance treatment with mirikizumab, a p19-directed IL-23 antibody: 52-week results in patients with moderately-to-severely active ulcerative colitis
The AMAC study, a Phase 2 randomised clinical trial, previously presented, investigated the effects of interleukin (IL)-23 blockade. IL-23 is a critical cytokine in the pathogenesis of Inflammatory Bowel Disease. Mirikizumab (miri), a p19-directed IL-23 antibody, demonstrated efficacy and was well tolerated during 12 weeks of induction treatment in the AMAC study. This presentation reported on the maintenance results through week 52 from this trial.
Patients who achieved a clinical response to miri at week 12 were re-randomised 1:1 into a double-blind maintenance period to receive miri 200 mg subcutaneously (SC) Q4W (n=47) or every 12 weeks (Q12W; n=46), and were treated through week 52. At baseline, 52.7% had previously received a biologic. At week 52, 46.8% (Q4W) and 37.0% (Q12W) were in clinical remission. Additionally, 80.9% (Q4W) and 76.1% (Q12W) had a clinical response, and 57.4% (Q4W) and 47.8% (Q12W) had an ES=0/1. Among those in clinical remission at week 12, 61.1% (Q4W) and 38.5% (Q12W) remained in clinical remission at week 52. Among those in clinical response (but not remission) at week 12, 37.9% (Q4W) and 36.4% (Q12W) achieved clinical remission at week 52.
In this trial, miri demonstrated durable efficacy (assessed by multiple measures) with no unexpected safety signals and few discontinuations due to AEs during the maintenance period. These results are useful as they demonstrate that a p19-directed IL-23 antibody may be an effective treatment as maintenance therapy in patients with moderately to severely active UC.


