Y-ECCO Literature Review: Andrea Centritto
Andrea Centritto
Point-of-care intestinal ultrasound in IBD patients: Disease management and diagnostic yield in a real-world cohort and proposal of a point-of-care algorithm
Bots S, De Voogd F, De Jong M, et al.
J Crohns Colitis 2022;16:606–615.
|
|
Introduction
Intestinal ultrasound (IUS) is an inexpensive, non-invasive, safe and repeatable, dynamic cross-sectional imaging technique for IBD. It has been demonstrated to be accurate and reliable both for initial diagnosis of IBD and for follow-up monitoring [1]. Huge advantages of IUS are that it does not need any prior preparation of the patient and provides a real-time result. IUS can be performed in various hospital settings, which makes it the only point-of-care (POC) imaging technique available today [2].
The impact of POC IUS on daily decision making and the evolution in its use over the years were evaluated in this retrospective study, which included two consecutive cohorts of IBD patients in a real-world outpatient setting. The first cohort of patients, included between January 2016 and July 2018, was compared with a second cohort collected between October 2019 and December 2019.
Key Findings
A total of 345 IUS were performed in 301 patients, of whom 242 had Crohn’s Disease (CD) and 59, Ulcerative Colitis (UC). For the bowel wall thickness, a cut-off of 2 mm and 3 mm was used for small bowel (SB) and colon, respectively.
Of the 345 IUS, 190 showed active disease, 113 showed no signs of inflammation, 37 showed uncertain disease and 5 were inconclusive. Seventy-three complications were detected. The reasons for uncertainty were minor findings, bowel gas, abdominal fat and complex surgical history.
In 207 cases (60%), the examination led to a change in treatment plan and in 122 (58.9%) additional investigations were necessary. Reasons for additional investigations were inadequacy of IUS, baseline evaluation before starting or follow-up of biologic treatment, stricture dilatation, evaluation of treatment response, extensive complications, inclusion in clinical trial, melaena and suspicion of malignancy.
In patients with CD, IUS was able to detect active disease in 67.6% of symptomatic and 54.3% of asymptomatic patients. In patients with UC, it did so in 57.1% of symptomatic and only 10% of asymptomatic patients.
IUS was compared with faecal calprotectin with three cut-offs: 50 µg/g, 150 µg/g and 250 µg/g.
IUS showed active disease in 71.0% of cases with FCP >50 µg/g versus 15.0% with FCP <50 µg/g, in 73.7% of cases with FCP >150 µg/g versus 24.1% with FCP <150 µg/g and in 79.6% of cases with FCP >250 µg/g versus 31.7% with FCP <250 µg/g.
As regards C-reactive protein (CRP), IUS showed active disease in 77.5% of patients with CRP >5 mg/L and in 51.4% of patients with CRP <5 mg/L.
IUS showed a strong correlation with additional endoscopy (p<0.0001), with presence or absence of disease activity being comparable in 44 out of 51 cases.
Comparison of 15 IUS and MRI examinations with a certain outcome (adequate image quality and no doubt over findings), and with MRI performed within 8 weeks after IUS, revealed that 75% of IUS examinations showed active disease comparable to MRI findings while 25.0% did not show active disease, unlike on MRI.
A comparison between the two cohorts was made. In total, 250 IUS were performed in the first cohort (January 2016–July 2018) and 95 in the second (October–December 2019). Comparing the same period of October to December in 2016 and 2019, it was found that 40 and 95 IUS, respectively, were performed. No differences in patient characteristics or symptoms were observed between the cohorts. However, the indications for IUS did differ: in the first cohort IUS was mostly used for confirmation of active disease and complications, whereas in the second it was more often employed for treatment response monitoring. Also, in the first cohort, fewer patients were treated with corticosteroids and biologics.
Conclusion
IUS has previously been revealed to be accurate in detecting inflammation in more than half of patients investigated, and to lead to a modification of the treatment plan in almost half of these patients. IUS findings in CD patients correlate less well with symptoms and biochemical findings than do IUS findings in UC patients, with a higher percentage of active disease being found in asymptomatic patients with normal biochemical markers [3,4].
There is a high correlation between IUS and endoscopy findings when IUS is considered adequate. Of course, some patients will need further investigations owing to lack of definite findings on IUS, and in such cases the symptoms and biochemical findings will drive decision making.
IUS has been demonstrated to be useful in diagnosing complications such as strictures and to help with decision making in these cases. Unfortunately, however, IUS is not helpful in distinguishing a fibrotic stricture from one with active disease and thus cannot aid management decisions in these cases.
Again, IUS has limited accuracy for examination of the rectum and proximal SB [5,6], and MRI has been confirmed to be more accurate for this purpose. However, at least for pelvic disease, transperineal IUS seems feasible and is accurate and non-invasive [7].
It is interesting that in this study by Bots et al., the use of IUS altered between 2016 and 2019. As mentioned above, whereas it was primarily used to confirm diagnosis and complications in the first cohort, in the second it was more frequently used for disease monitoring and confirmation of disease remission. More sonographers will have been trained over these years, and overall there was an increase in the use of IUS and a decrease in the number of MRI examinations in the second cohort.
This study seems to indicate that extra investigations were mostly necessary when the IUS was inadequate, with extensive complications and with suspicion of proximal SB and rectal disease. On the other hand, IUS may be useful when endoscopy is unsuccessful [8].
The authors proposed an algorithm for the use of IUS in both CD and UC like the one suggested by Allocca et al. [9]. According to this algorithm (Fig. 1), IUS should be performed in the presence of symptoms and/or elevated biomarkers in both diseases, but in addition, a monitoring IUS is appropriate in patients with CD even when these criteria are not met, bearing in mind the poor correlation between inflammation and symptoms/biomarkers. When an adequate IUS is performed, this could be sufficient for treatment planning. However, if IUS is inadequate, or in cases of CD with suspicion of extensive disease/complications, further investigations may be considered. Ultimately, IUS follow-up is suggested at 8 weeks for UC and 3 months for CD, taking into account the longer resolution of inflammation in CD.
A limitation of this study is its retrospective nature, as a result of which Other limitations are a patient selection bias resulting from IUS becoming more widely available and accepted and the lack of a control group. Strengths of the study are the large size of the cohort analysed and the real-world setting.
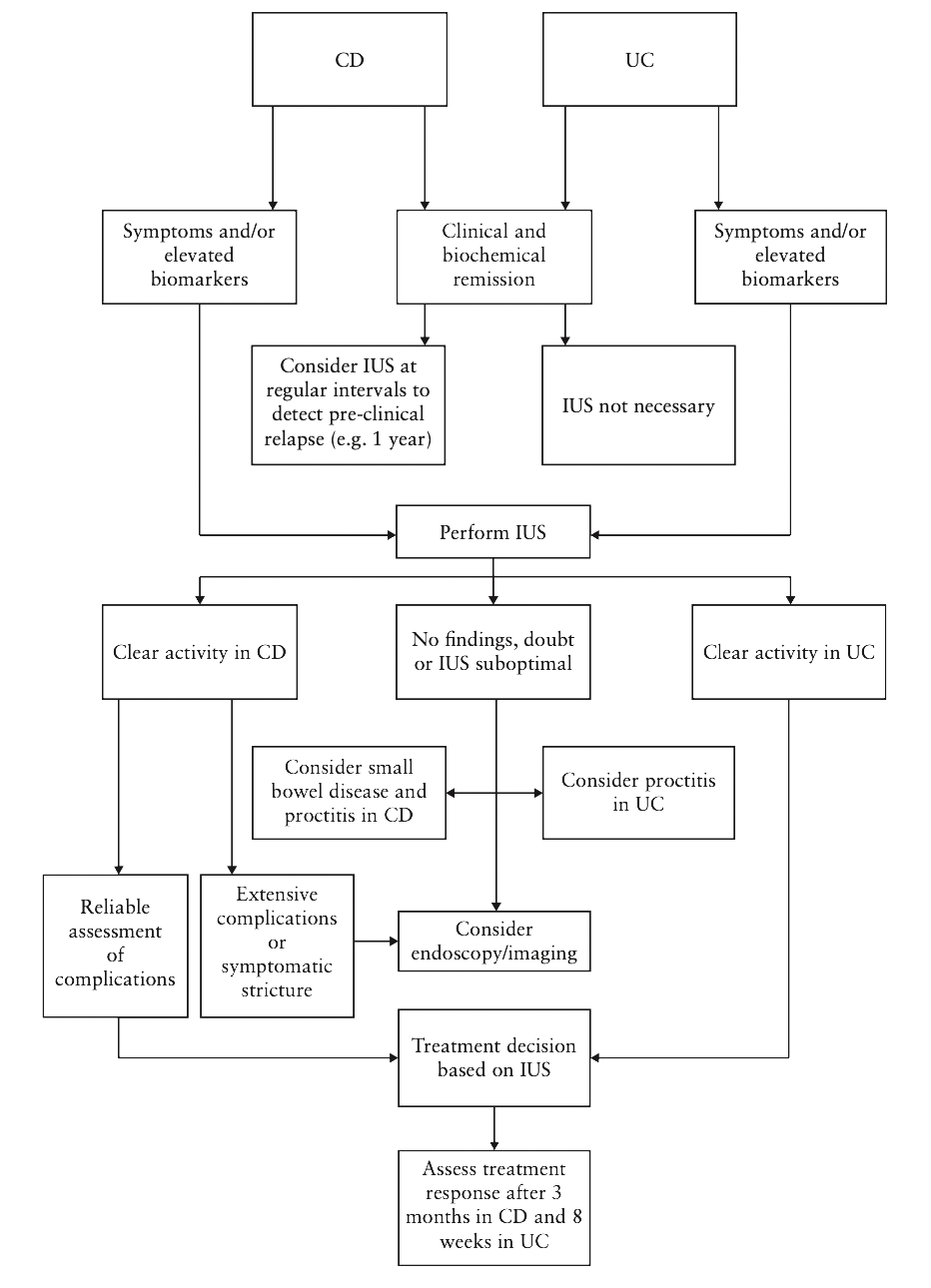
Figure 1. Proposal of a POC IUS algorithm
References
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in Inflammatory Bowel Disease. J Crohns Colitis 2019;13:144–64.
- de Voogd FAE, Verstockt B, Maaser C, Gecse KB. Point-of-care intestinal ultrasonography in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2021;18:209–10.
- Novak K, Tanyingoh D, Petersen F, et al. Clinic-based point of care transabdominal ultrasound for monitoring Crohn’s disease: impact on clinical decision making. J Crohns Colitis 2015;9:795–801.
- Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 2002;122:512–30.
- Smith RL, Taylor KM, Friedman AB, Gibson RN, Gibson PR. Systematic review: Clinical utility of gastrointestinal ultrasound in the diagnosis, assessment and management of patients with ulcerative colitis. J Crohns Colitis 2020;14:465–79.
- Taylor SA, Mallett S, Bhatnagar G, et al.; METRIC study investigators. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease [METRIC]: a multicentre trial. Lancet Gastroenterol Hepatol 2018;3:548–58.
- Sagami S, Kobayashi T, Aihara K, et al. Transperineal ultrasound predicts endoscopic and histological healing in ulcerative colitis. Aliment Pharmacol Ther 2020;51:1373–83.
- Wilkens R, Novak KL, Lebeuf-Taylor E, Wilson SR. Impact of intestinal ultrasound on classification and management of Crohn’s disease patients with inconclusive colonoscopy. Can J Gastroenterol Hepatol 2016;2016:8745972.
- Allocca M, Furfaro F, Fiorino G, Peyrin-Biroulet L, Danese S. Point-of-care ultrasound in inflammatory bowel disease. J Crohns Colitis 2021;15:143–51.
Andrea Centritto - Short Biography
My name is Andrea Centritto, I’m a gastroenterologist working as senior fellow in Inflamatory Bowel Disease at the Guy’s and St. Thomas’ NHS Trust in London. I am a qualified small bowel sonographer and my fellowship’s project is to implement the SB ultrasound service in our Trust. My main interest is IBD and within it the development of IUS as daily tool of diagnosis and monitoring, and endoscopy.



